Basic QC Practices
The 2025 Great Global QC Survey USA Results
In 2025, have QC practices in the USA gotten better or worse? The results are in.
The 2025 USA QC Survey Results: Quality is getting more out-of-control
Sten Westgard, MS
August 2025
[This survey was completed with the support and partnership of Thermo Fisher MAS controls.]
In 2025, have QC practices in the US improved or declined?
We surveyed US laboratories in 2017 and 2021 about their quality control practices. We did it again in 2025.
Asia: 2025 Great Global QC Survey Results: Asia Breakout - Westgard QC
Europe: The 2025 Great Global QC Survey: Europe in isolation - Westgard QC
Middle-East: 2025 Great Global QC Survey Results: Middle East - Westgard QC
Latin and South America: 2025 Great Global QC Survey Results: South and Latin America - Westgard QC
Africa: 2025 Great Global QC Survey Results: Africa - Westgard QC
USA: https://westgard.com/qc-applications/basic-qc-practices/2025-qc-survey-usa.html
All of it together: https://westgard.com/qc-applications/basic-qc-practices/2025-global-qc-survey.html
The demographics of the participants
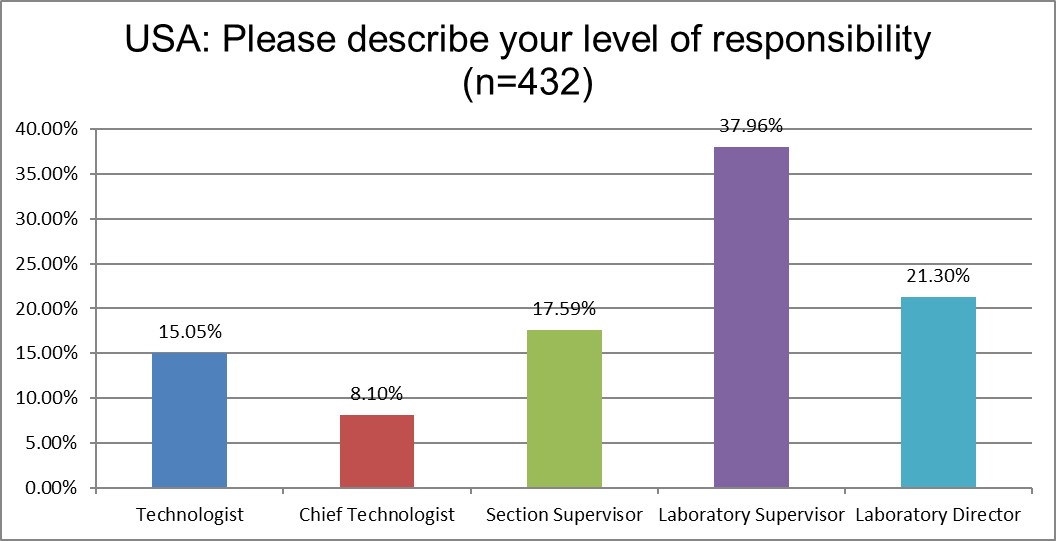
We saw in increase in the responses from supervisors, a decrease in the number of responses from technologists.
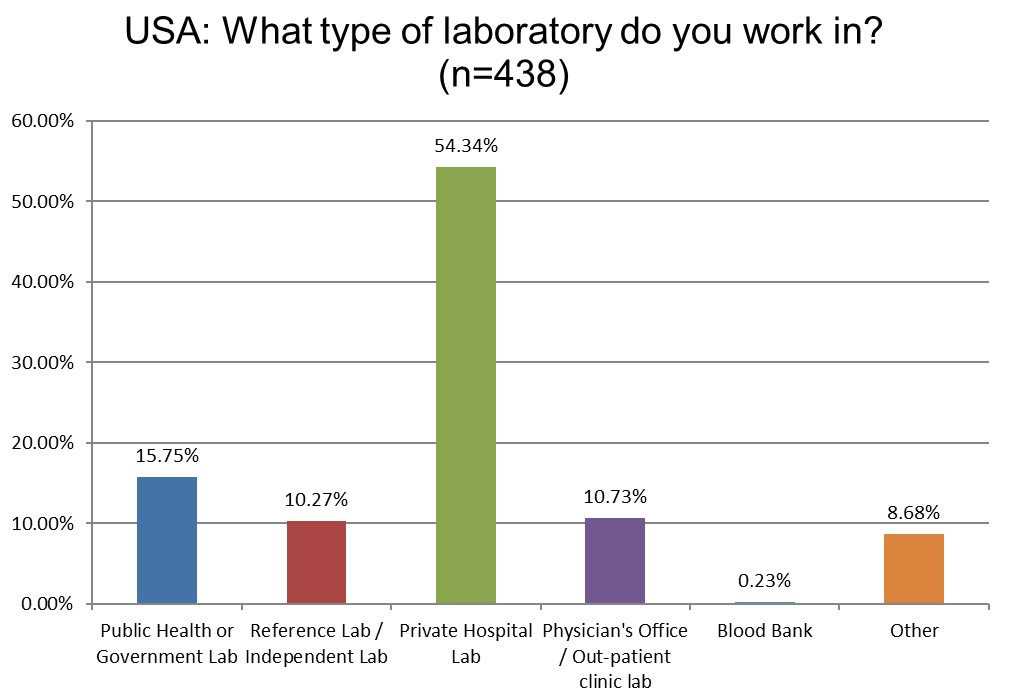
Again, a majority of the responses are coming from private hospitals and laboratories, reflecting the structure of the US healthcare system.
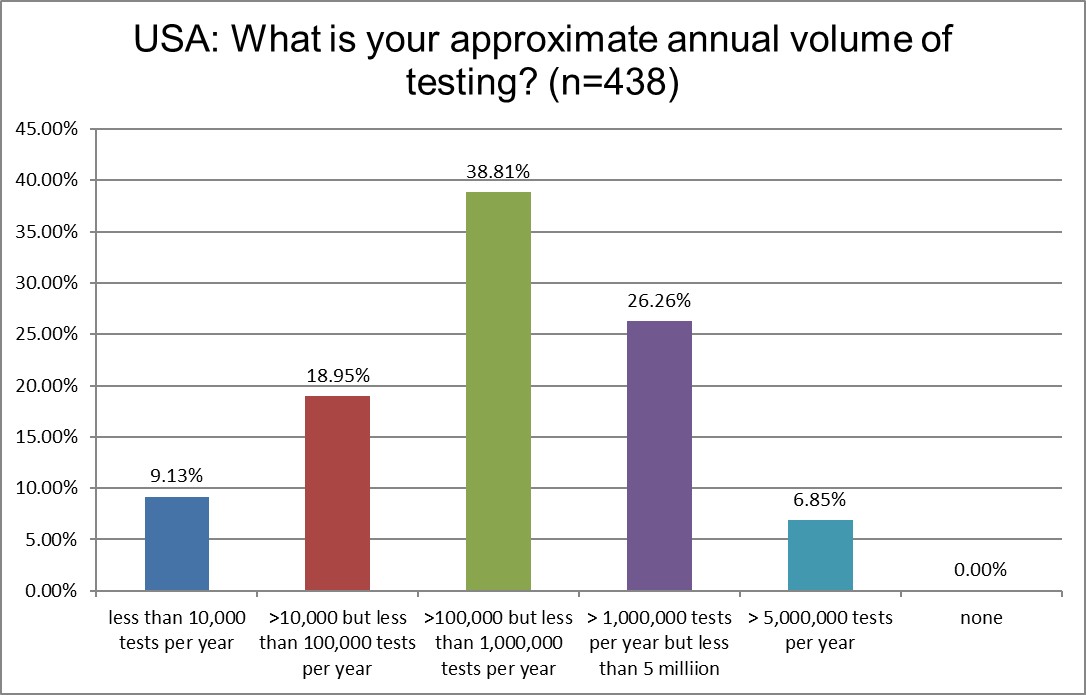
The skew of this bell curve is toward the bigger but not the mega testing volumes. There is just a slight increase in the number of mega-labs responding to the survey in 2025.
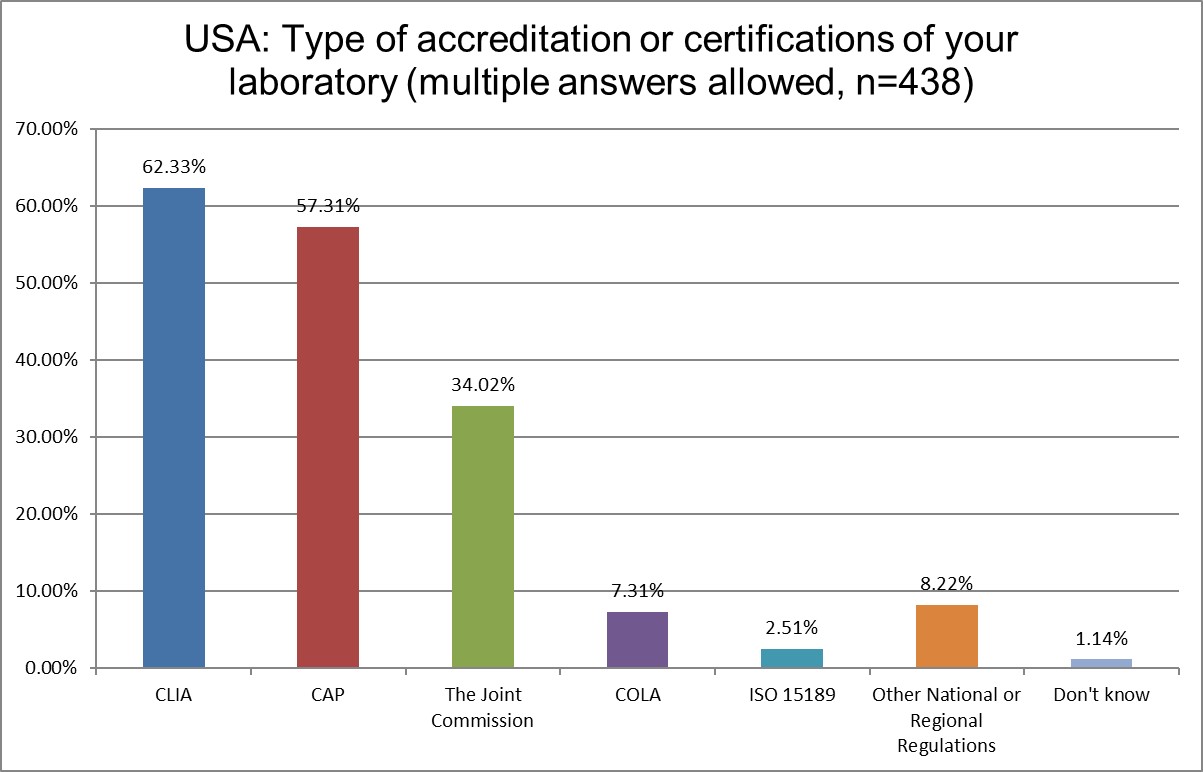
The ISO 15189 standard is basically unimplemented here in the US, with CLIA regulations the law of the land, but the percentage of ISO 15189 labs double from 1.25% to 2.5% since 2025. CAP labs still dominate over TJC and COLA accreditation.
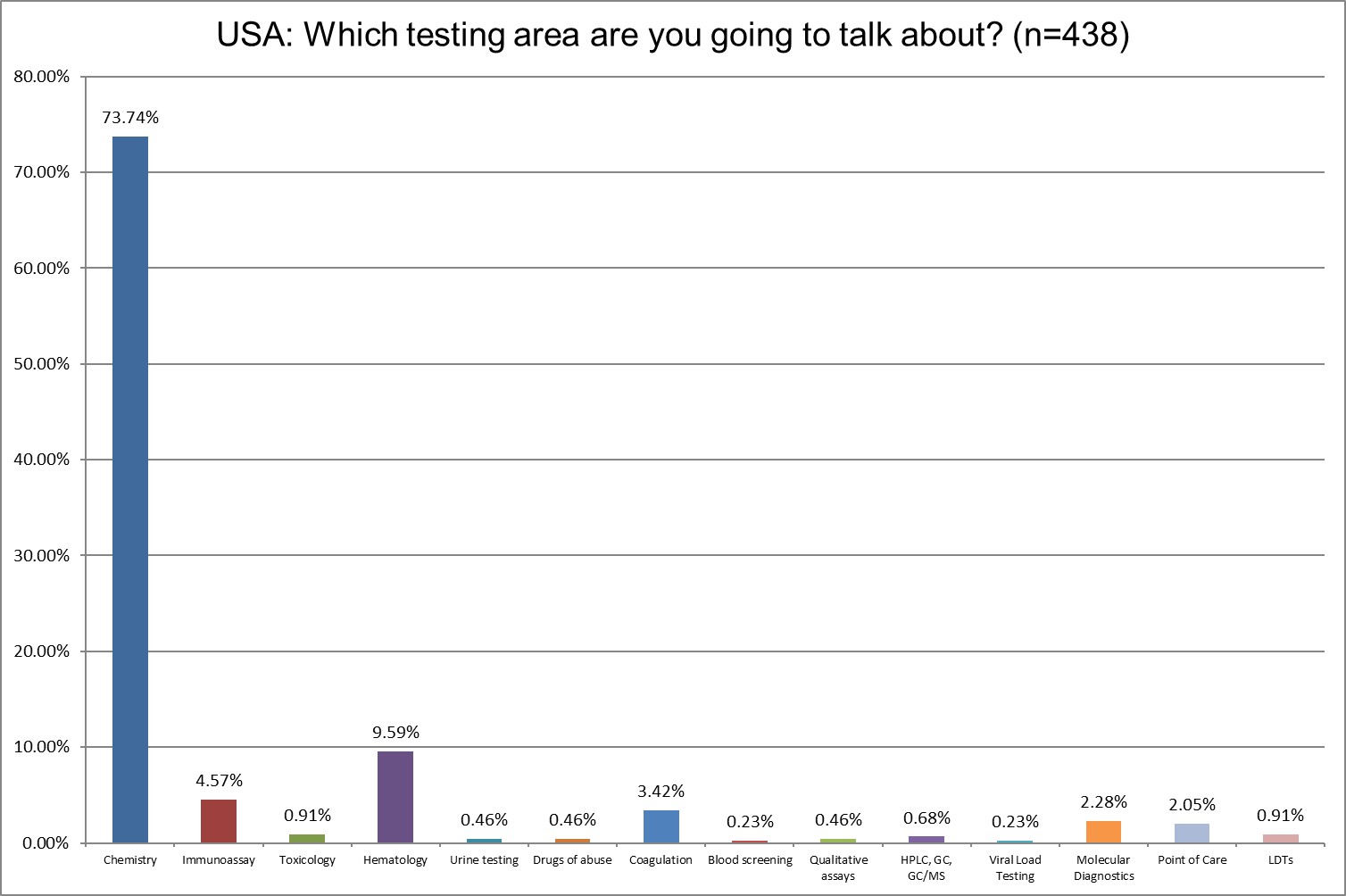
The vast majority of the survey reponses focus on chemistry testing. We will in fact break that out specifically in future analysis.
The QC Set Up
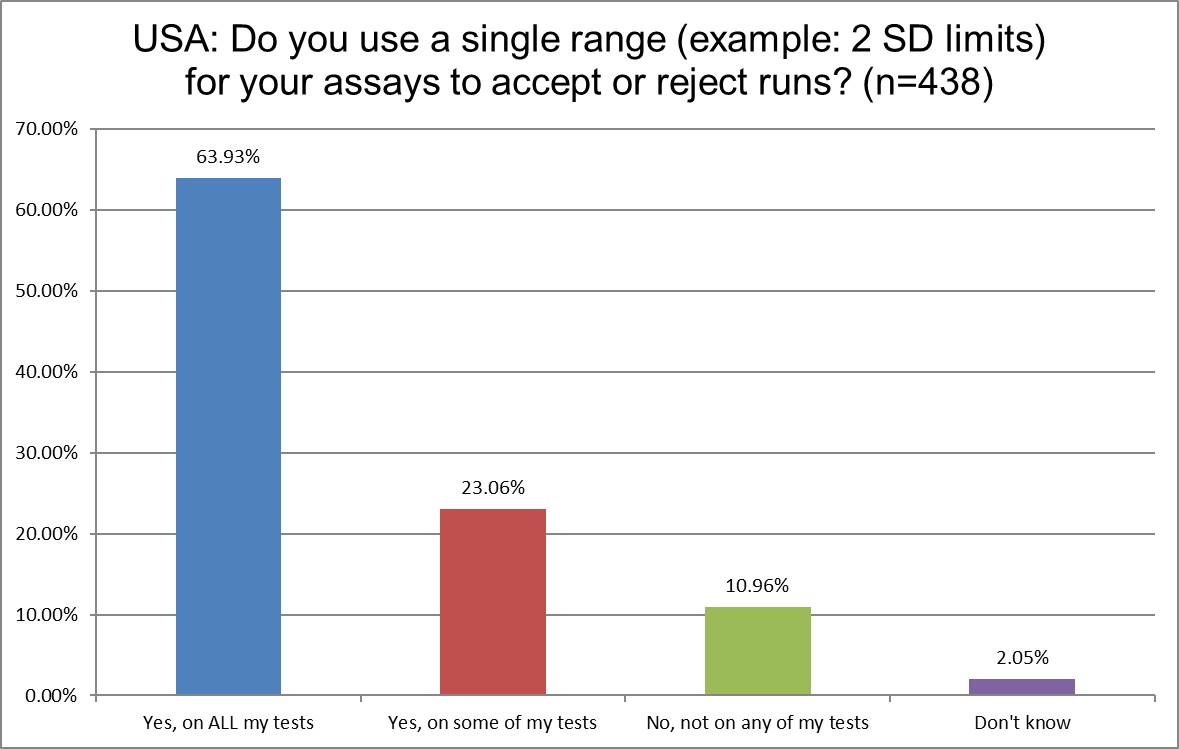
The use of 2 SD on all testing has declined by 10% in the US. However, the overall presence of 2 SD (all + some) in US labs has actually slightly increased. We've been using these limits for over half a century. It's straight up statistical fact that they generate false rejections rates of 9% (for 2 controls) and 14% (for 3 controls). And yet, somehow, more labs are using them. A continuation of decline we've seen over 3 surveys.
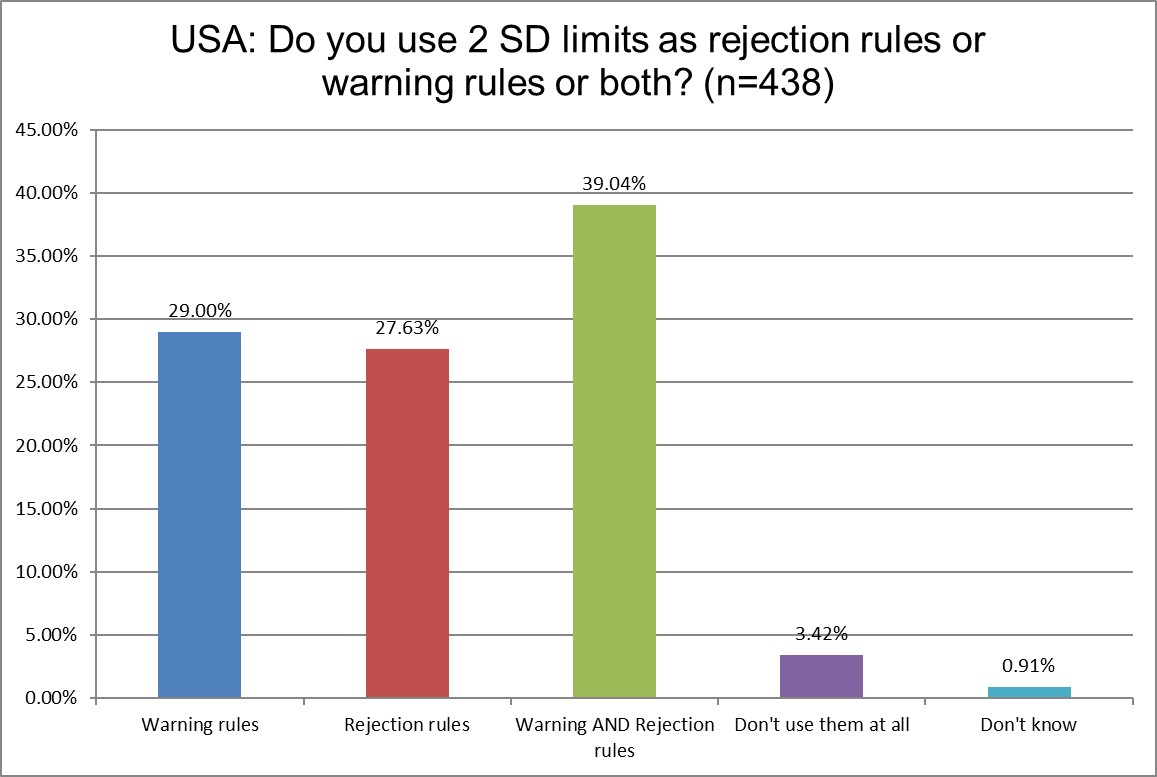
This is a new question we asked for the 2025 survey. We've seen that some labs continue to use 2 SD limits and justify them as "only a warning." But the data tells us more than 2/3rds of US labs are using 2 SD for rejection rules or both warning and rejection rules. That means the false rejection rate is not being balanced out by being ignored. As we will see later, these practices feed into high repeat rates for controls.
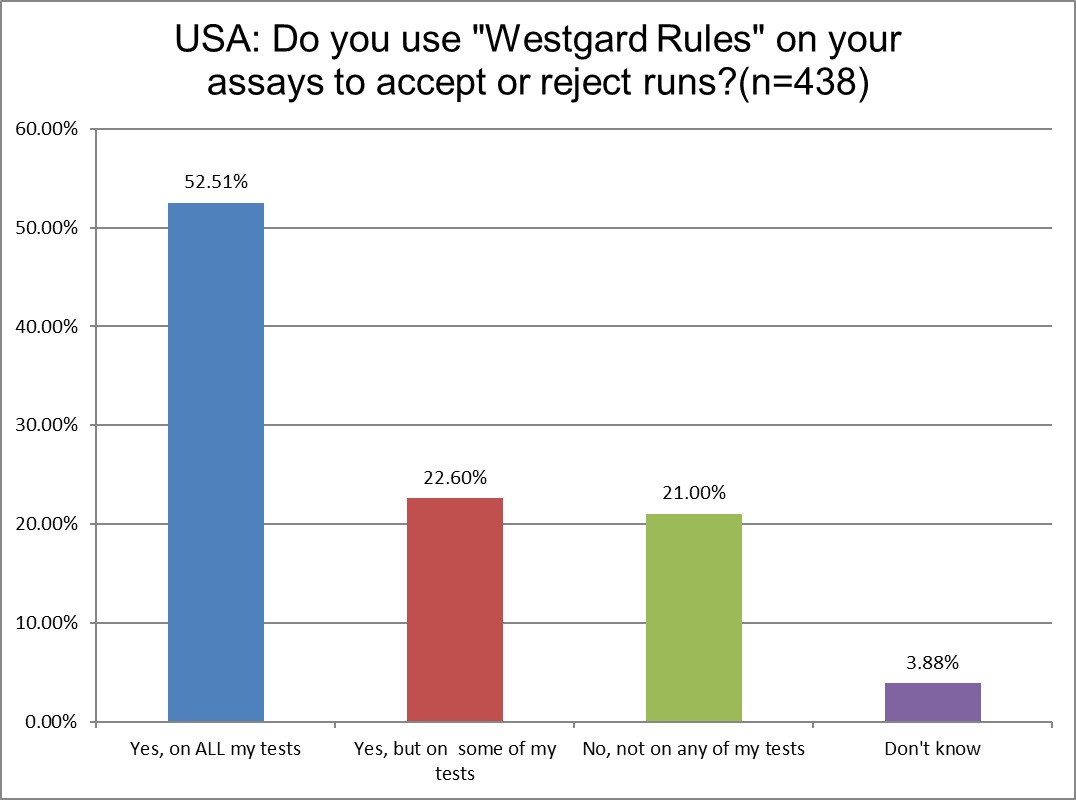
"Westgard Rules" are still in use, to some extent, in over 90% of labs globally. But in the US, the use is steady at about 75%. We've seen a slight decline in the use of Westgard Rules on all tests, from about 58% to 52%. Since we've been actively recommending the strategic selective use of Westgard Rules since 1992, this is not an unwelcome development.
.
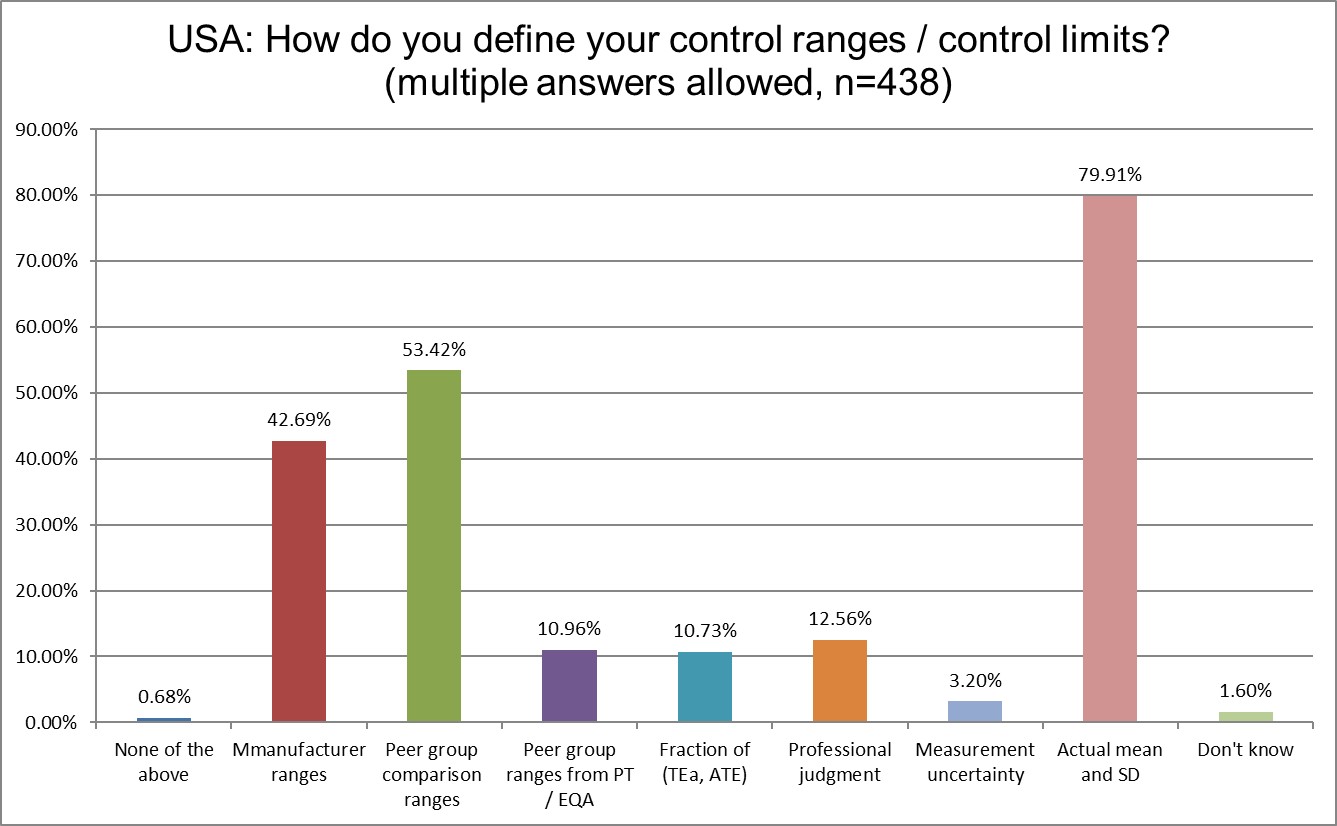
Happily, we see that the use of actual mean and SD has increased from 74% (2021) to almost 80%. Also a decrease in the use of manufacturer ranges from 50% (2021) to 42.7%. Use of peer group ranges also increased from 48% (2021) to 53%, an increase not so positive.
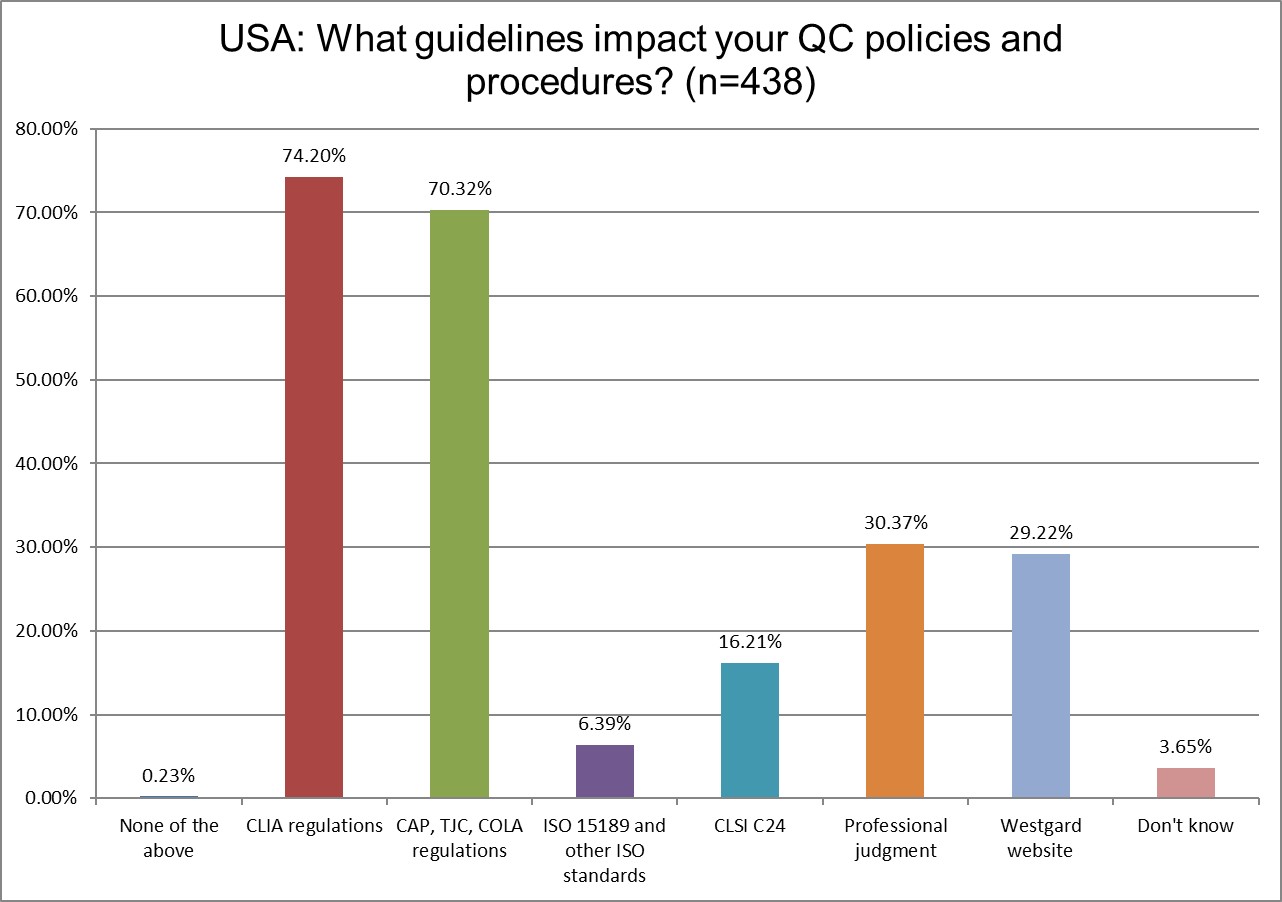
Interestingly, there was a dip in reliance on CLSI guidelines bewteen 2017, 2021 and 2025. The decline has been erased now.
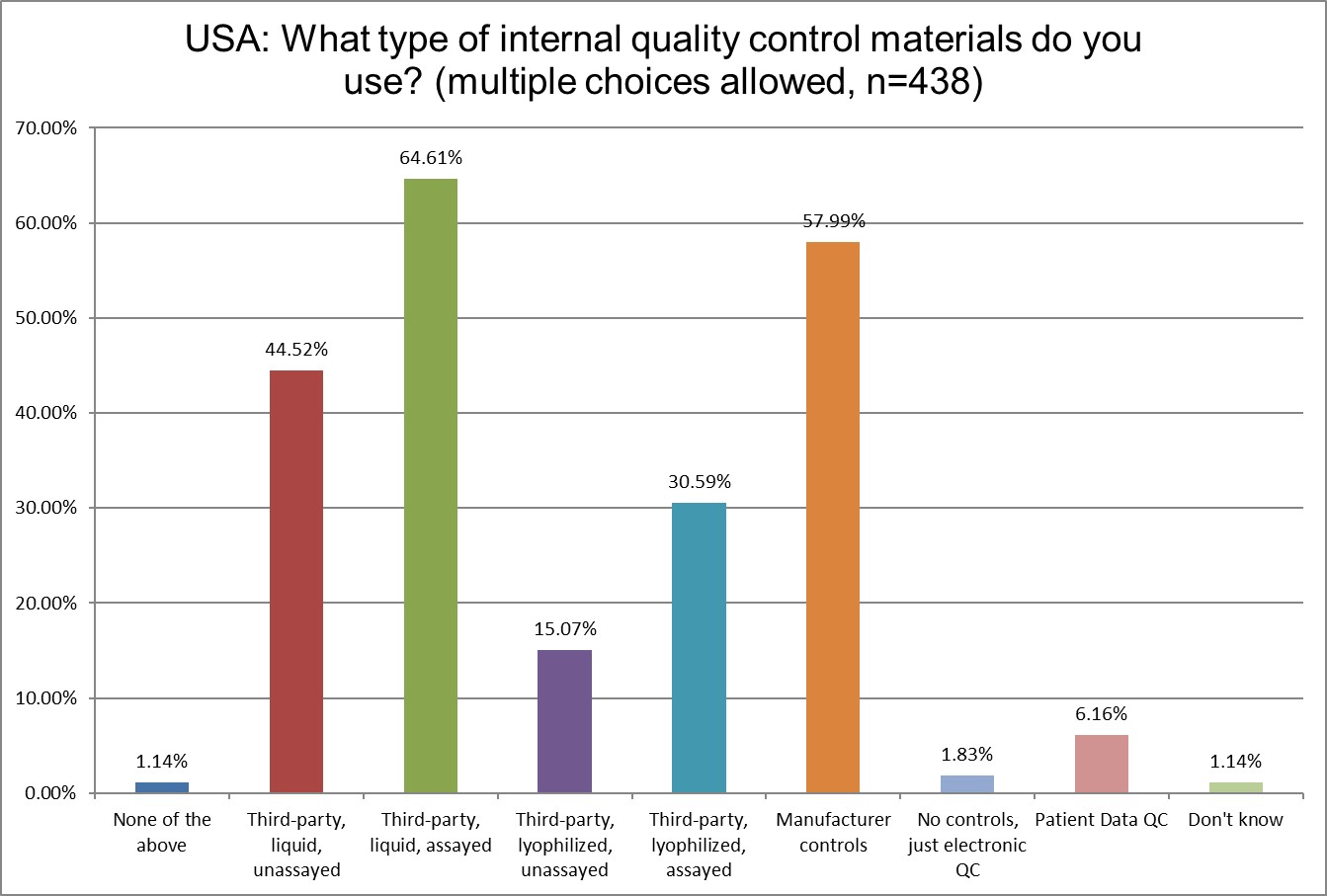
The use of 3rd party liquid, unassayed controls has not changed from 2021 to 2025. Slightly more labs are using 3rd party liquid, assayed controls (60.6% in 2021 to 64.6% in 2025). The use of manufacturer controls has declined from 62.53% to 57.9%, which is unusual, compared to other areas of the world. Patient data QC, however, has fallen almost in half, from 11% in 2021 down to 6.16% in 2025.
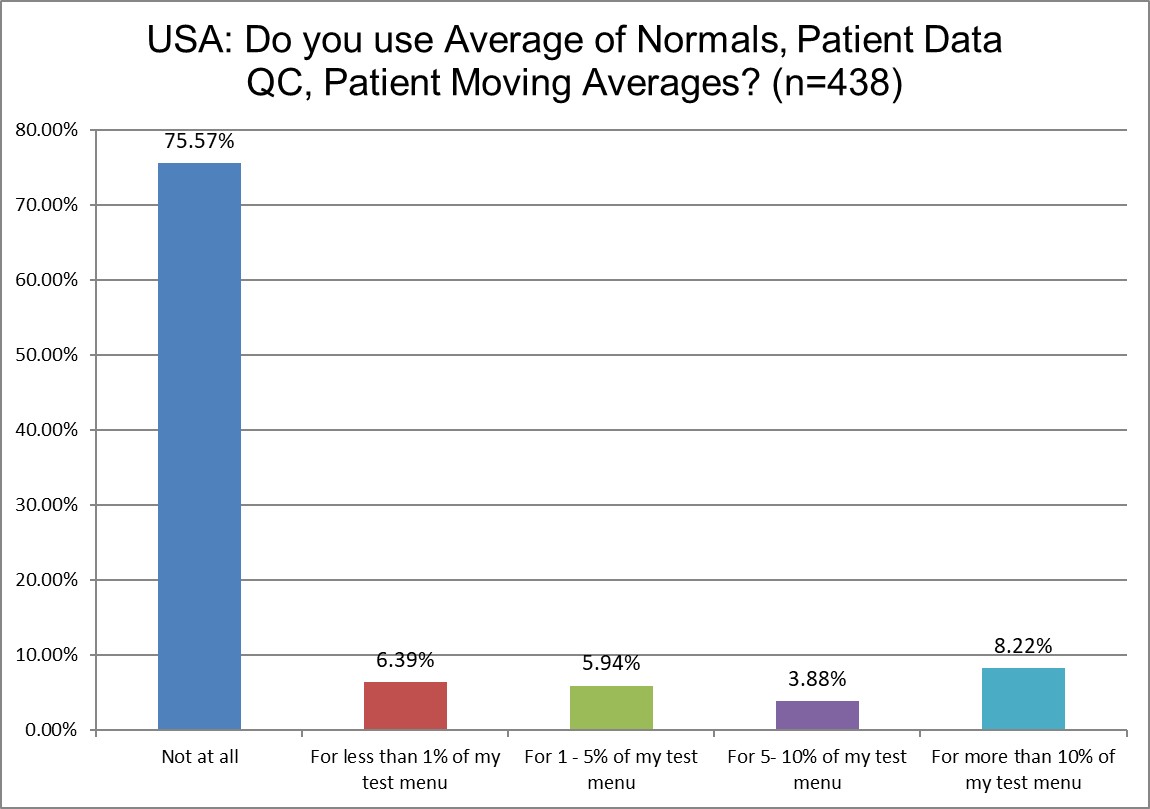
When we asked labs more specifically if they use patient data QC (or anything that fits under that umbrella term), the number of labs that don't use it at all declined slightly from 78.79% in 2021 to 75.75% in 2025. However, less than 9% of labs use the technique for a significant percentage of their testing menu. 90% of labs with 90% of their menus are using traditional QC to monitor errors. Despite considerable hype, promotion, papers, and conference presentations, patient data QC remains a boutique technique, one used very lightly by even the most enthusiastic of labs. It always sounds very exciting, but it's not the solution to our QC crises.
The Real Practice of Running Controls
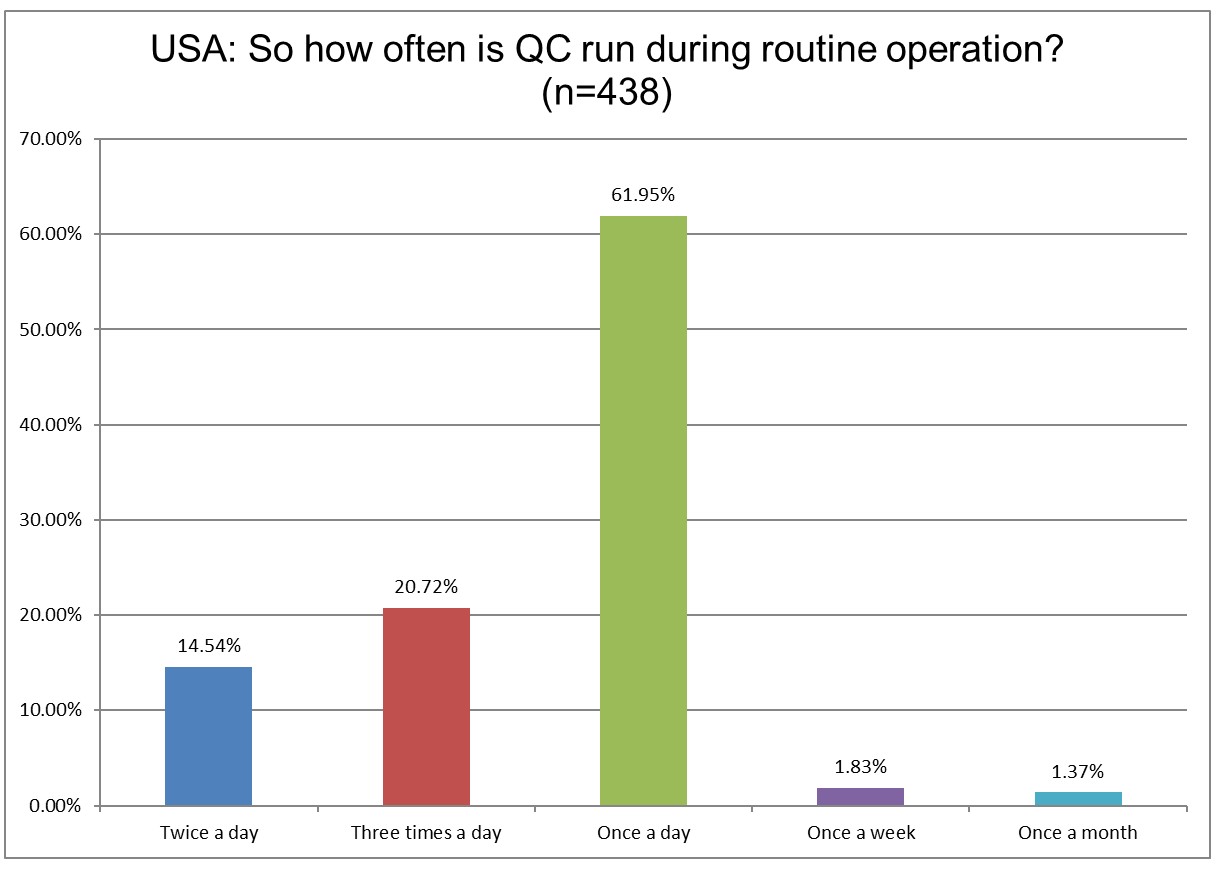
US labs are running less QC than they were 4 years ago. Running once a day QC increased, while running twice or three times a day has declined. The biggest decline occurred with 3x a day (28.28% in 2021, now 20.72% in 2025).
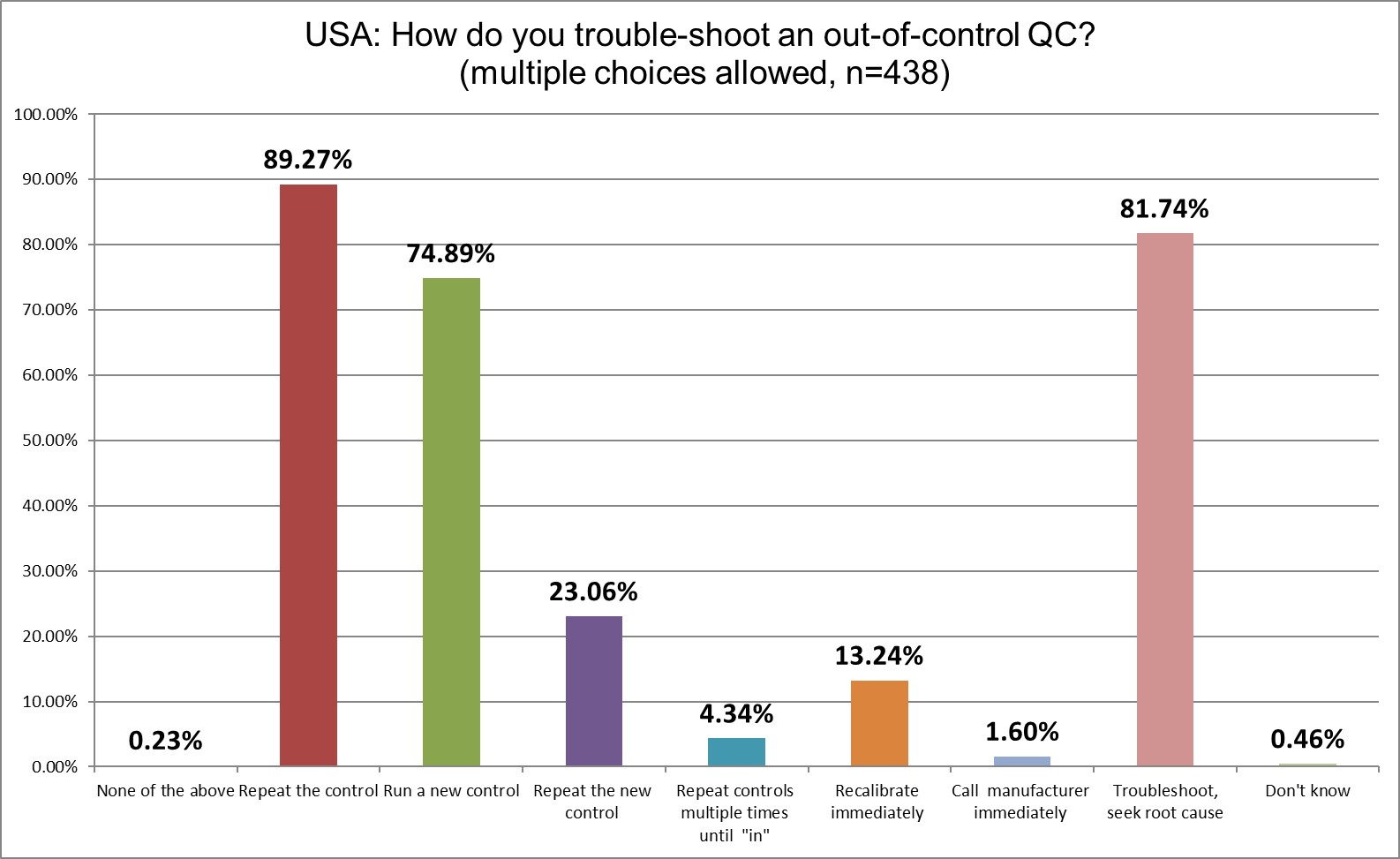
Interestingly, the percentage of labs repeating the control has barely changed (89.9% in 2021, now 89.27% in 2025).
Slightly fewer labs will run a new control after that (74.89% in 2025 vs. 79.8% in 2021). Over 4% of labs now say they keep repeating as much as necessary to get "in" - vs. zero percent in 2021. A worrying increase, but perhaps just an increase in honesty.
So the US retains the dubious distinction as the country where labs repeat controls more than anywhere else in the world. As we have long noted, this is not the act of a clinical facility, this is the act of a casino.
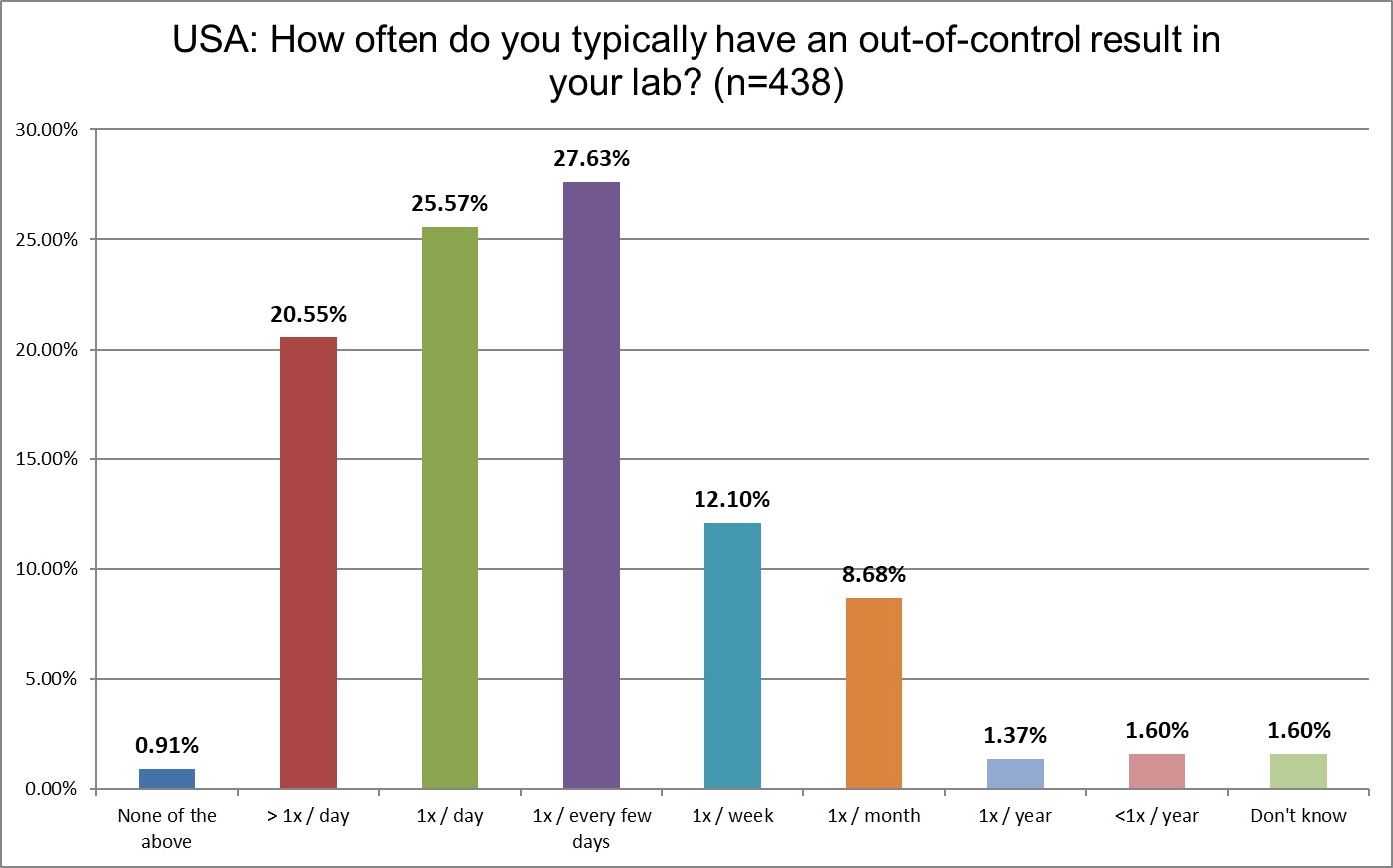
An even more disturbing finding in our survey is that US labs are experiencing significantly more oocs (out-of-control events) than ever before.
In 2021, 13% of US labs said they were ooc more than once a day. Now that's 20.55% in 2025. In 2021, 16% US labs said they were ooc once day. Now that's 25.57%. These are major increases in errors. In 2021, 29% of US labs were ooc every day. Now in 2025 that's increased to more than 46%. We're getting close of a majority of US labs being ooc every day. As we said earlier, this is a consequence of using false-alarm-prone 2 SD limits.
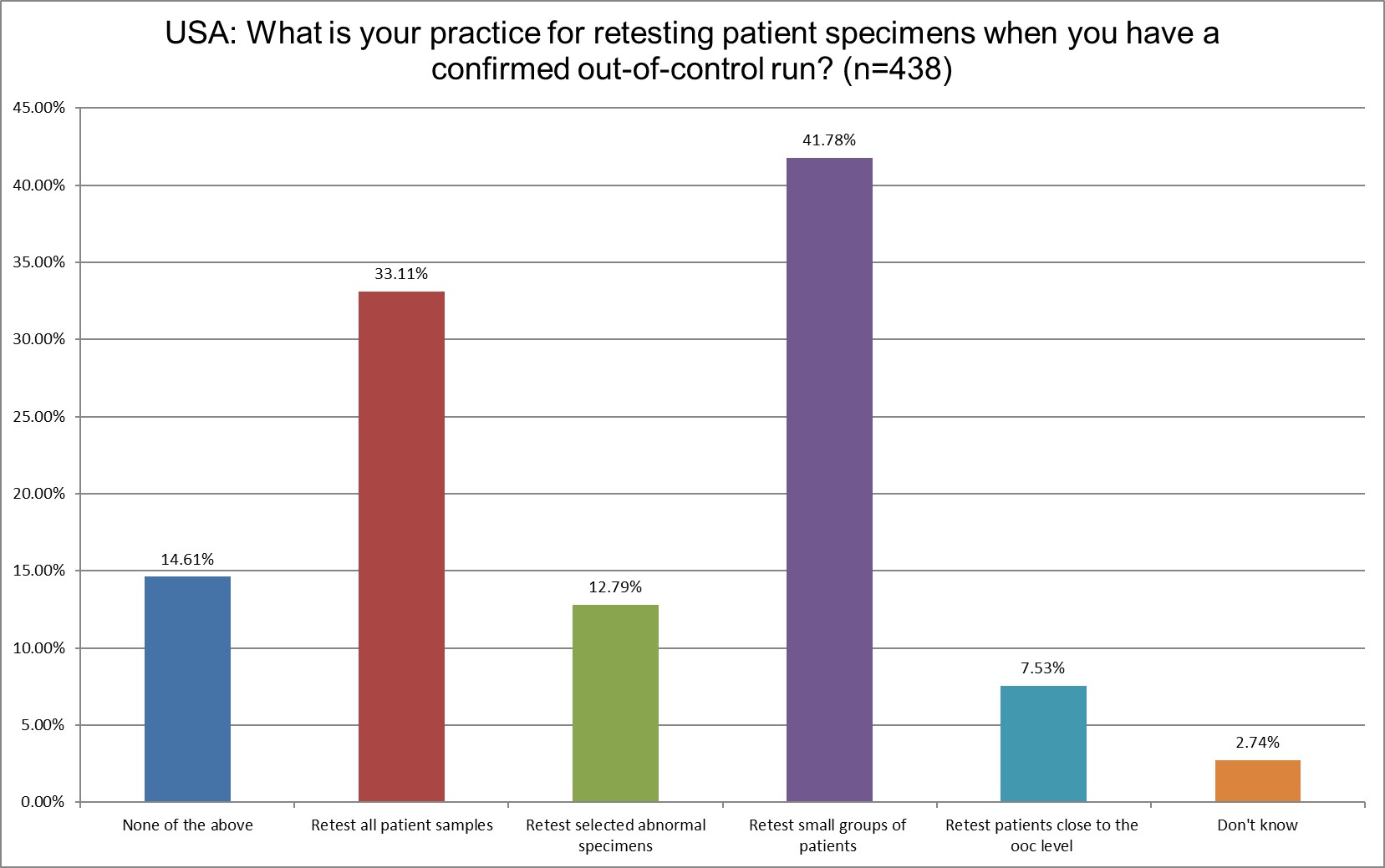
Only a third of US labs will retest all patient samples when there is an out of control run. The retesting of small groups of patients has increased from 34.34% in 2021 to 41.78% in 2025. That's the most significant change in practices.
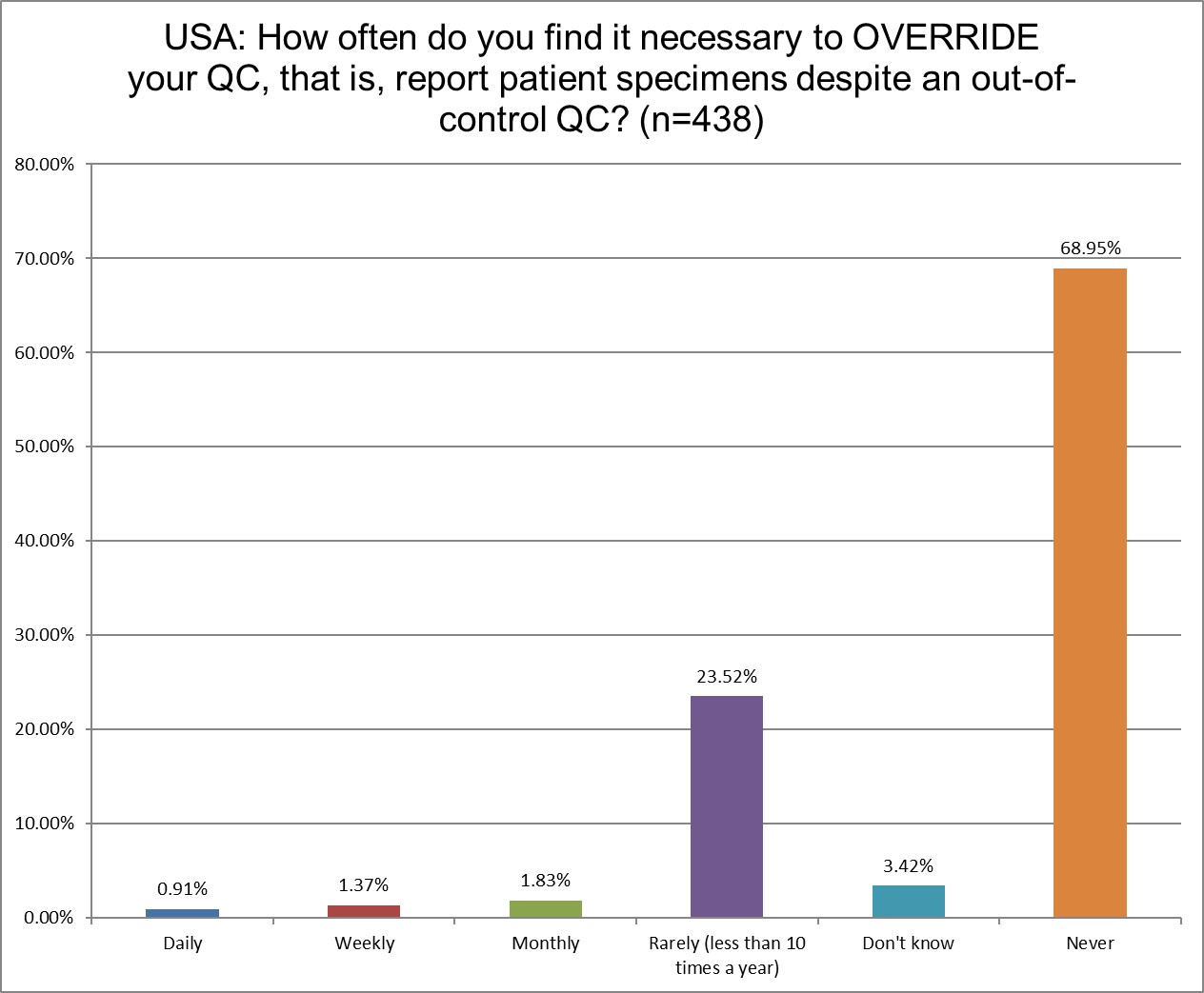
The good news is that the number of US labs that send out test results even when there's an out of control run is very, very small.
The Final Overview
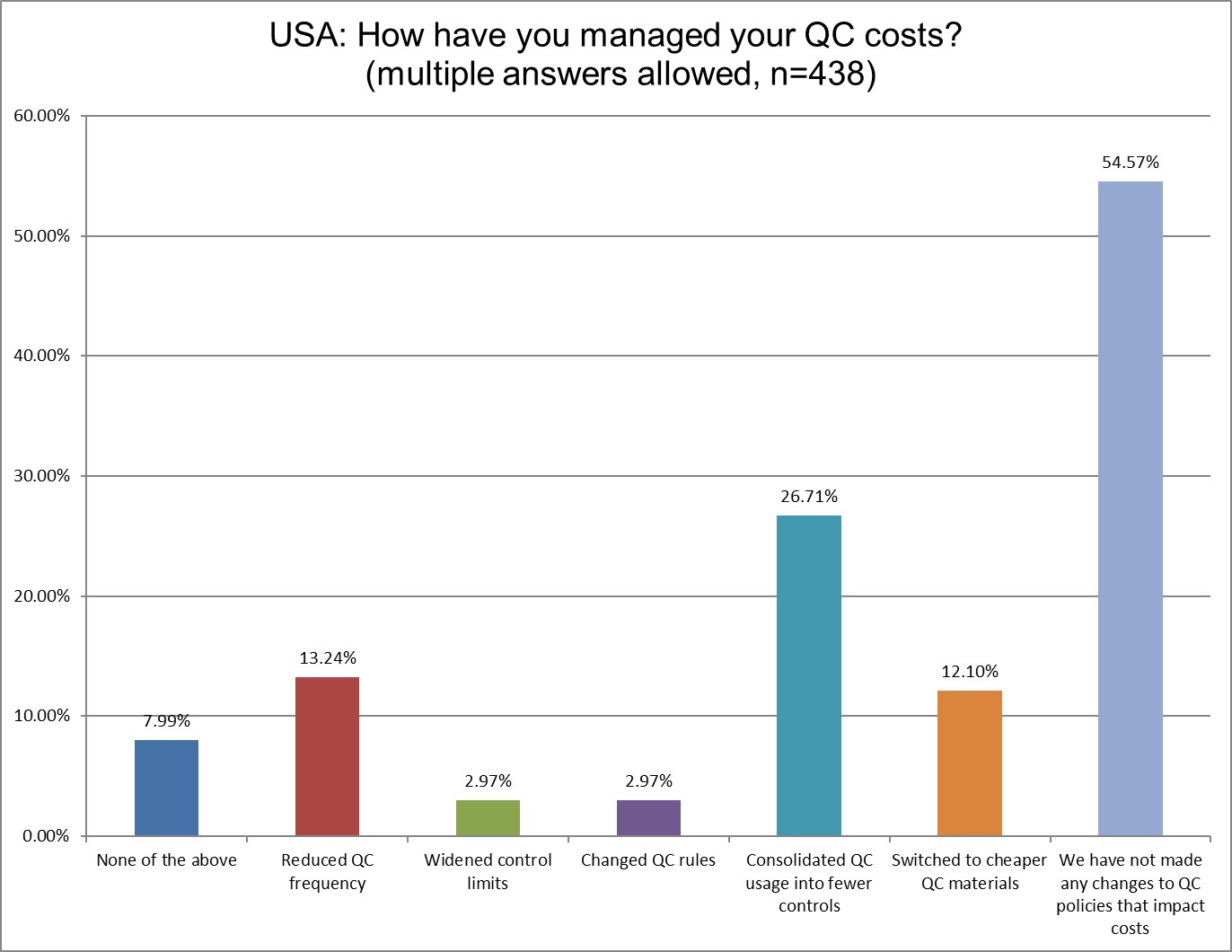
More US labs have attempted to grapple with their QC costs in the last few years. In 2021, 69.79% of US labs said they hadn't done anything. In 2025, now that's only 54.57%. The number of labs that have consolidated QC into fewer controls has more than doubled (11.46% in 2021 to 26.71% in 2025). The number of US labs that have switched to cheaper controls has tripled (4.17% in 2021 to 12.1% in 2025). Labs have also reduced QC frequency (9.38% in 2021 to 13.24% in 2025).
Still, for all this progress, a majority of US labs have still done nothing to change how they do QC and how much they spend on it.
