Basic QC Practices
2025 Great Global QC Survey: ISO and CLIA labs
In 2025, the Westgard Great Global Survey assessed the state of QC practices. In addition to regional differences, we looked at the differences between CLIA accredited labs and ISO 15189 labs. Do different standards mean different QC practices?
The 2025 Global QC Survey Results: Labs big and small
Sten Westgard, MS
October 2025
[This survey was completed with the support and partnership of Thermo Fisher MAS controls.]
In 2025, have QC practices around the world improved or declined?
We surveyed laboratories in 2017 and 2021 about their quality control practices. We did it again in 2025.
We got over 1,280 complete, qualified responses, which break down as follows:
- Africa 118 responses
- Asia 289 responses (note that we include everywhere from India to Australia within this group)
- Europe 143 responses
- Latin and South America 114 responses
- Middle-east 146 responses
- United States and Puerto Rico 440 responses
Asia: 2025 Great Global QC Survey Results: Asia Breakout - Westgard QC
Europe: The 2025 Great Global QC Survey: Europe in isolation - Westgard QC
Middle-East: 2025 Great Global QC Survey Results: Middle East - Westgard QC
Latin and South America: 2025 Great Global QC Survey Results: South and Latin America - Westgard QC
Africa: 2025 Great Global QC Survey Results: Africa - Westgard QC
USA: https://westgard.com/qc-applications/basic-qc-practices/2025-qc-survey-usa.html
All of it together: https://westgard.com/qc-applications/basic-qc-practices/2025-global-qc-survey.html
While we've completed regional analyses, we decided to look deeper at the differences between high volume and low volume laboratories.
- CLIA, CAP, and TJC accredited laboratories 575 responses
- ISO 15189 accredited laboratories 468 responses
Now the danger of this kind of analysis is that it could simply replicate the regional difference between the US and Europe. But in the US we only had 440 responses, so the group has some additional international labs that are CAP-accredited or TJC-accredited. And the international adoption of ISO 15189 is clearly represented. Europe only has 143 responses, but the ISO 15189 group is 468.
So we'll see if there is any practical difference in QC between these two groups. Let's dive right in.
The QC Set Up
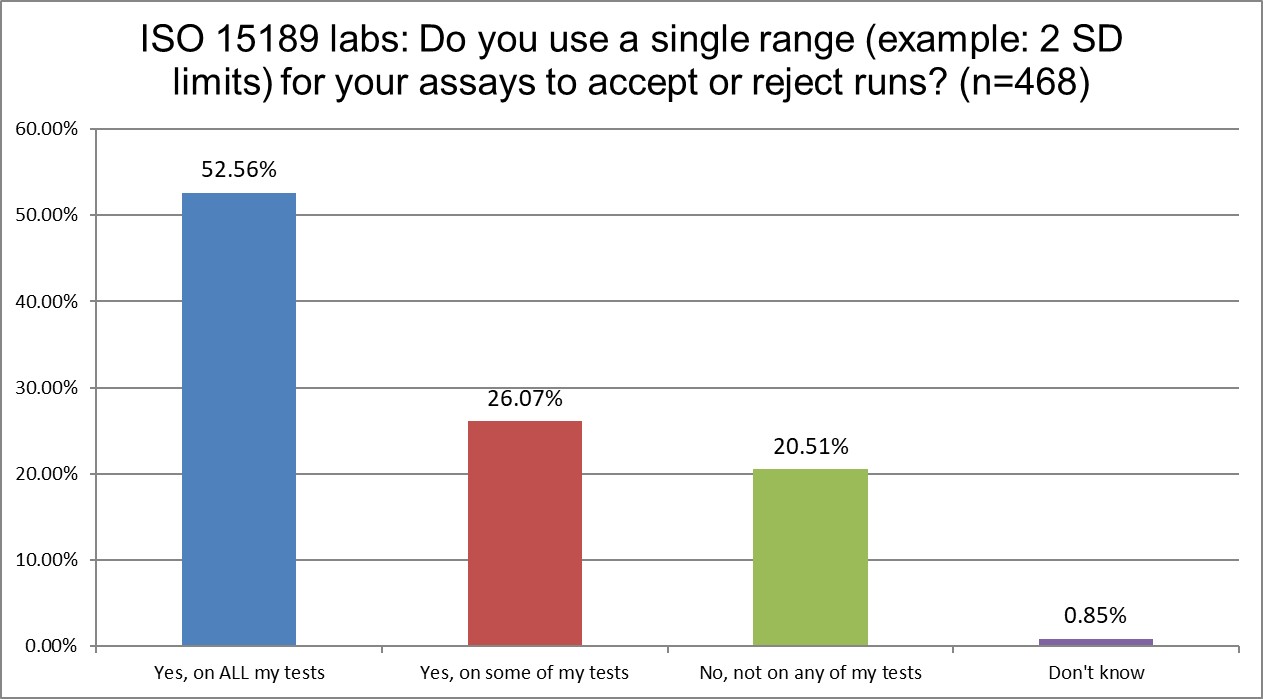
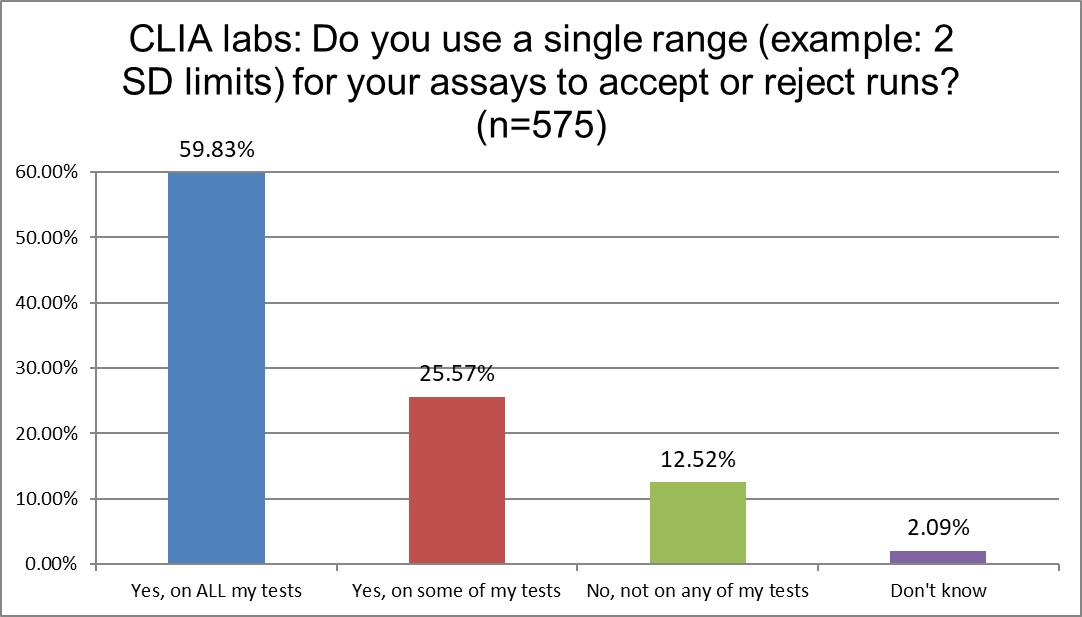
The use of 2 SD on all testing is somewhat consistent across labs ISO and CLIA. A majority of the labs in both groups use 2 SD on everything. About a qurter of each group uses 2 sd on some tests. The only big difference is that more ISO labs (>20%) vs CLIA labs (>12%) don't use 2 SD at all. But you can't say either standard is greatly impacting this QC habit.
Warning, Rejection, or Both?
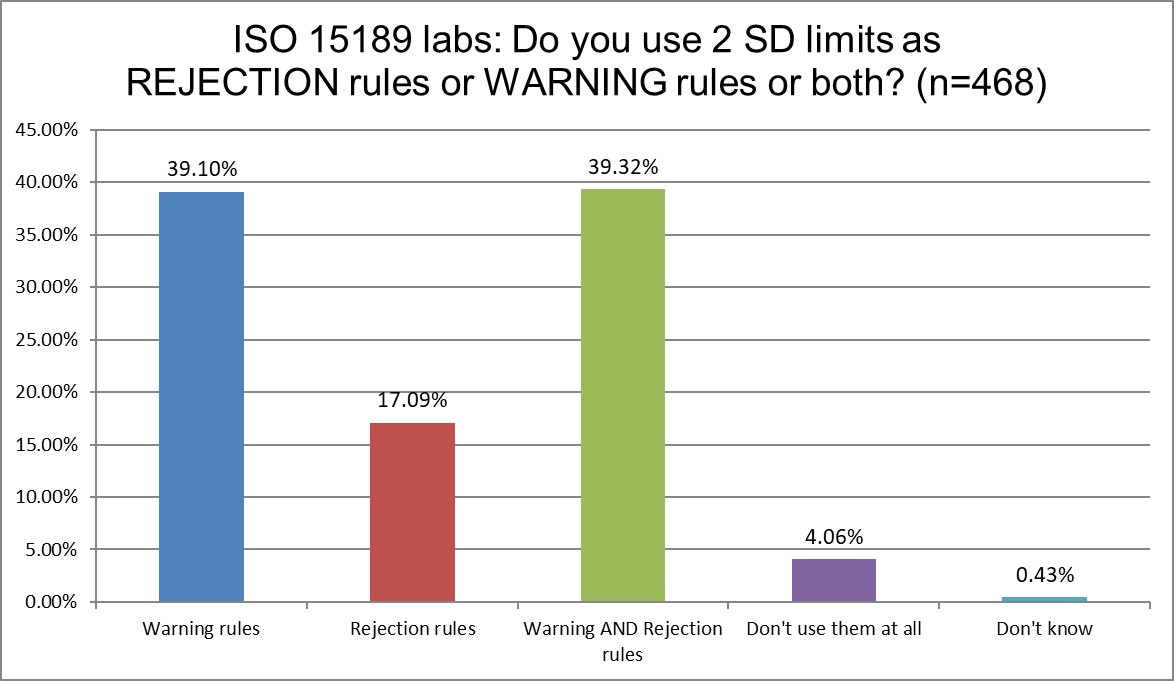
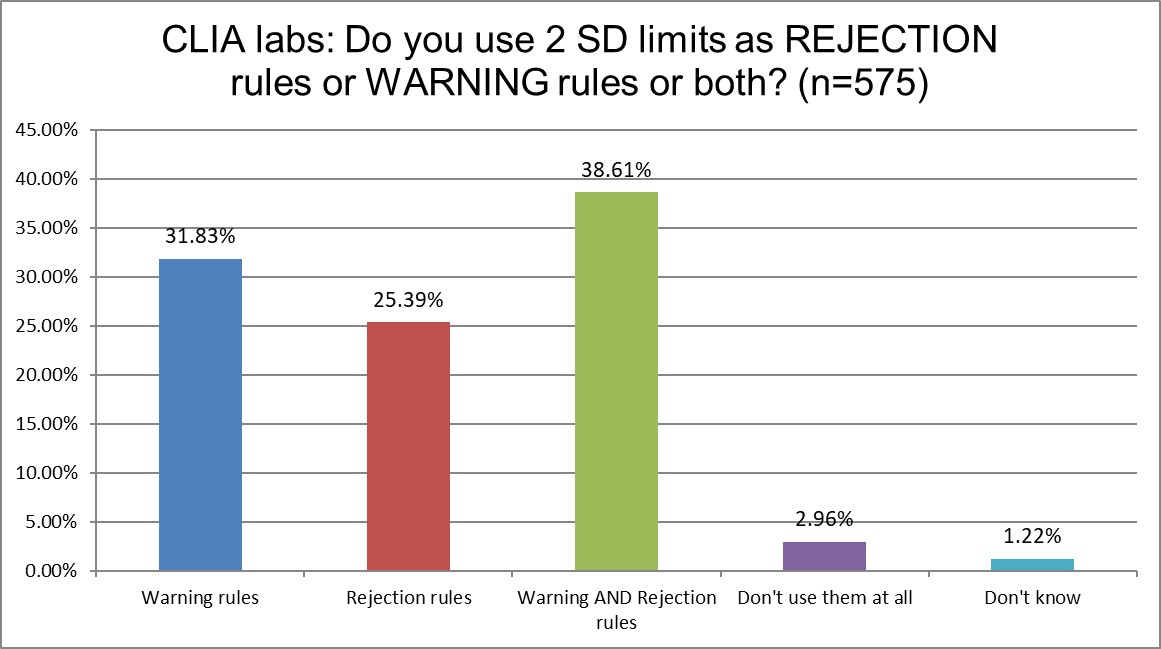
ISO and CLIA labs are again pretty similar in how they implement the 2 SD limits. About a third use them as warning rules, about a third use them as both rejection and warning rules. The difference is that ISO labs use them a bit more for warning rules, and CLIA use them a bit more for rejection rules.
"Westgard Rules" - do ISO or CLIA mandate or encourage them?
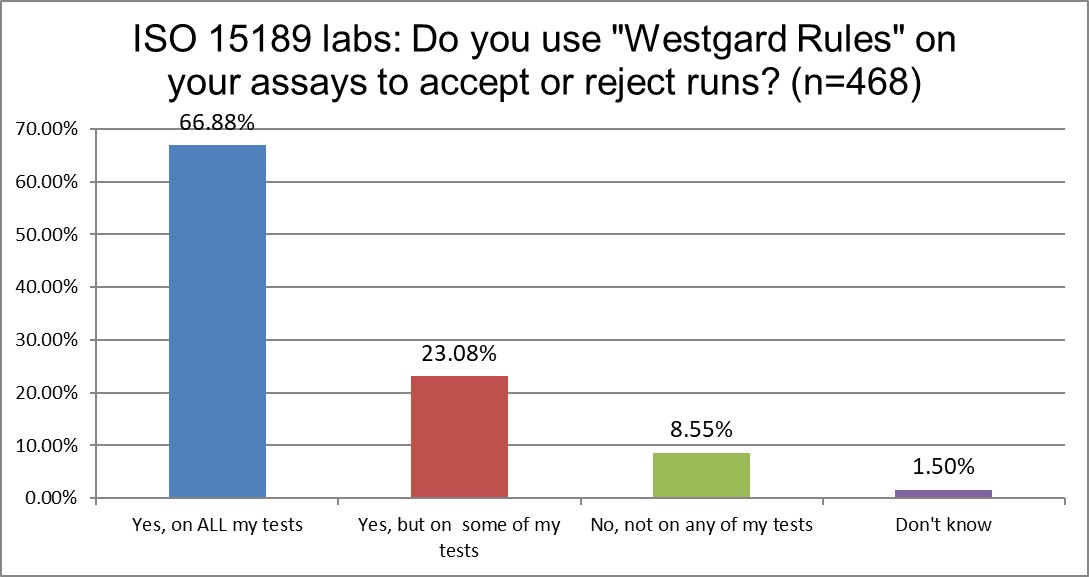
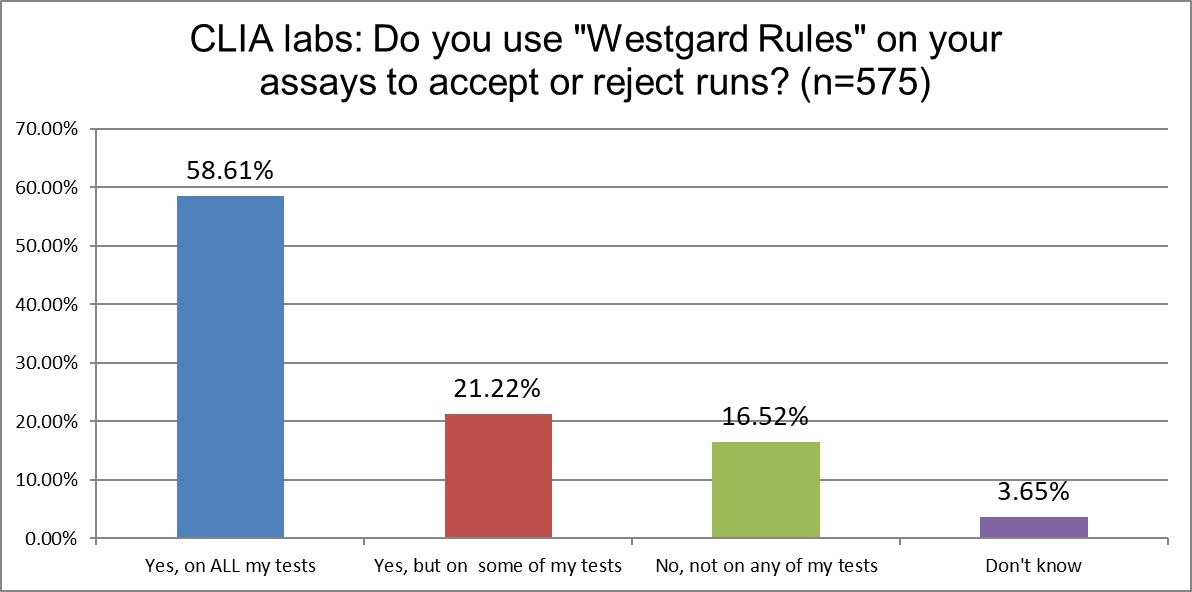
ISO labs use "Westgard Rules" on all tests more (67% vs 59%) than CLIA labs. They both use the rules on some tests at about the same rate. CLIA labs are twice as more likely not to use them at all. That might still reflect the bias in the USA (familiarity breeds contempt) against the Westgard Rules.
Are mean and SD choices influenced by accreditation type?
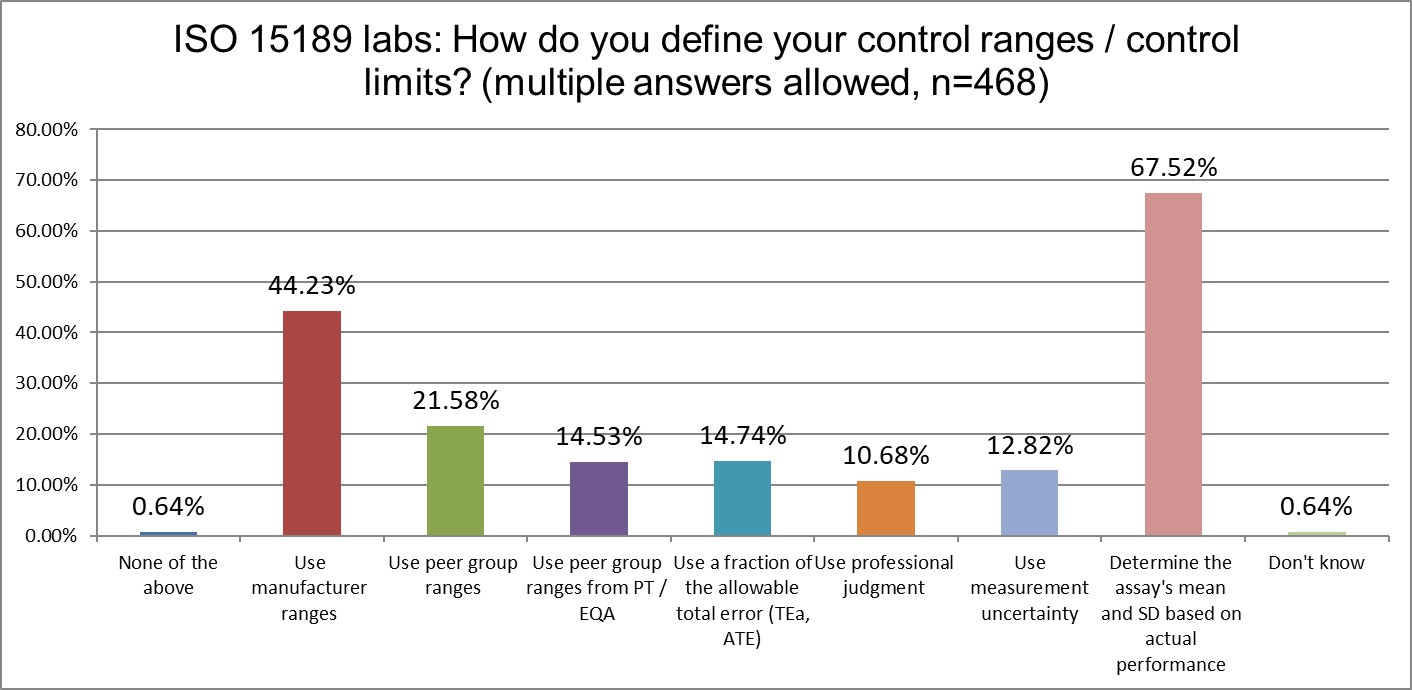
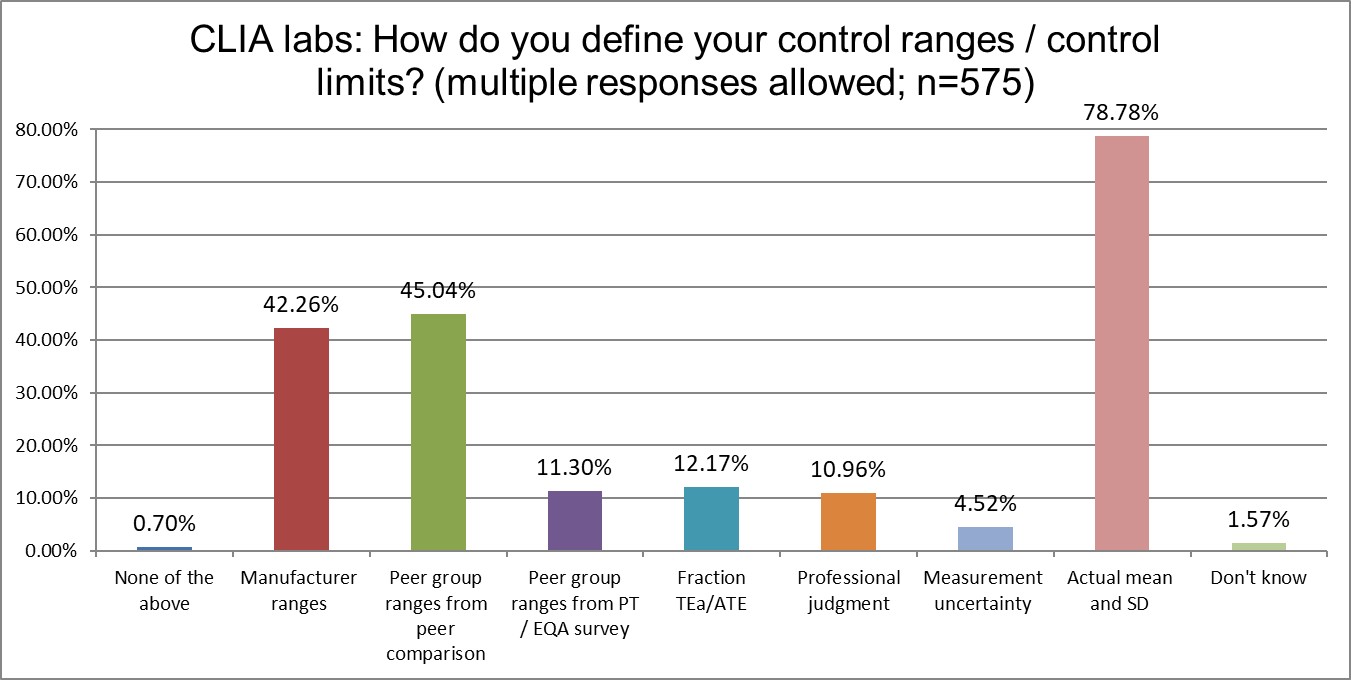
Both ISO and CLIA labs are most likely to calculate their own mean and SD (best practice). There is some divergence on the second-most popular choice: ISO labs like to use manufacturer ranges, while CLIA labs like to use peer group ranges (followed very closely by manufacturer ranges). CLIA labs are also more likely to calculate their own mean and SD.
What kind of controls are being used in ISO and CLIA labs?
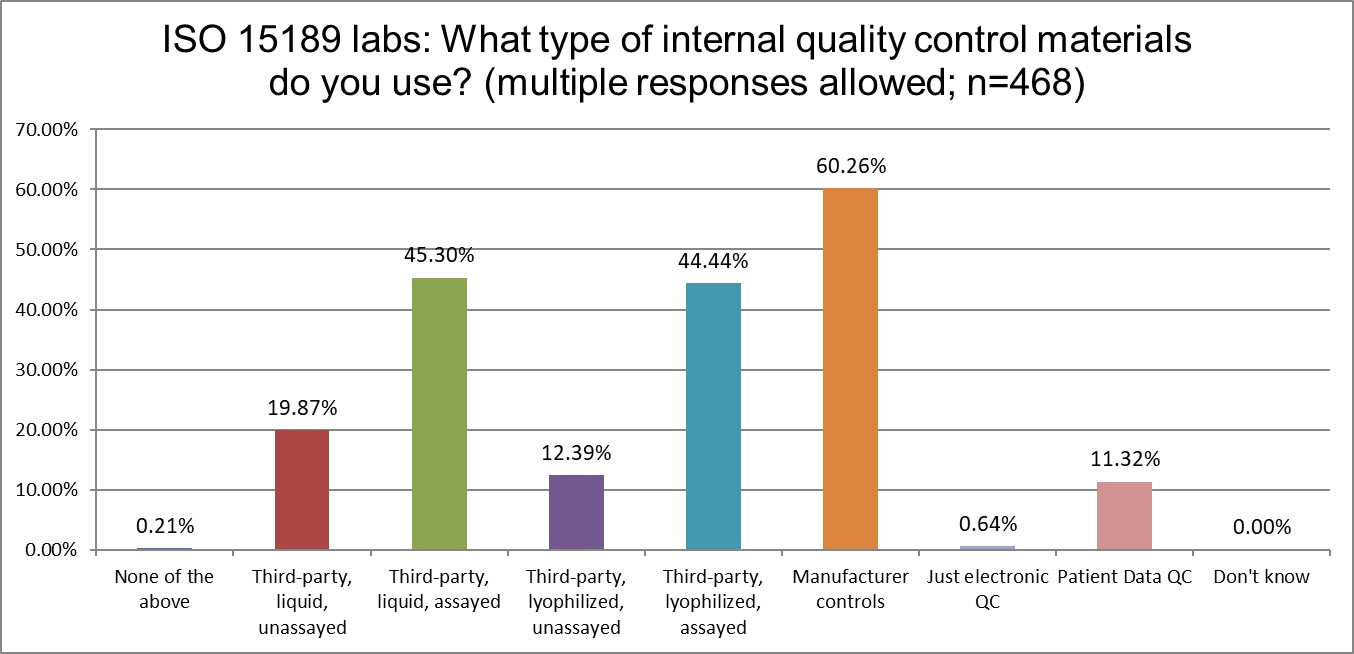
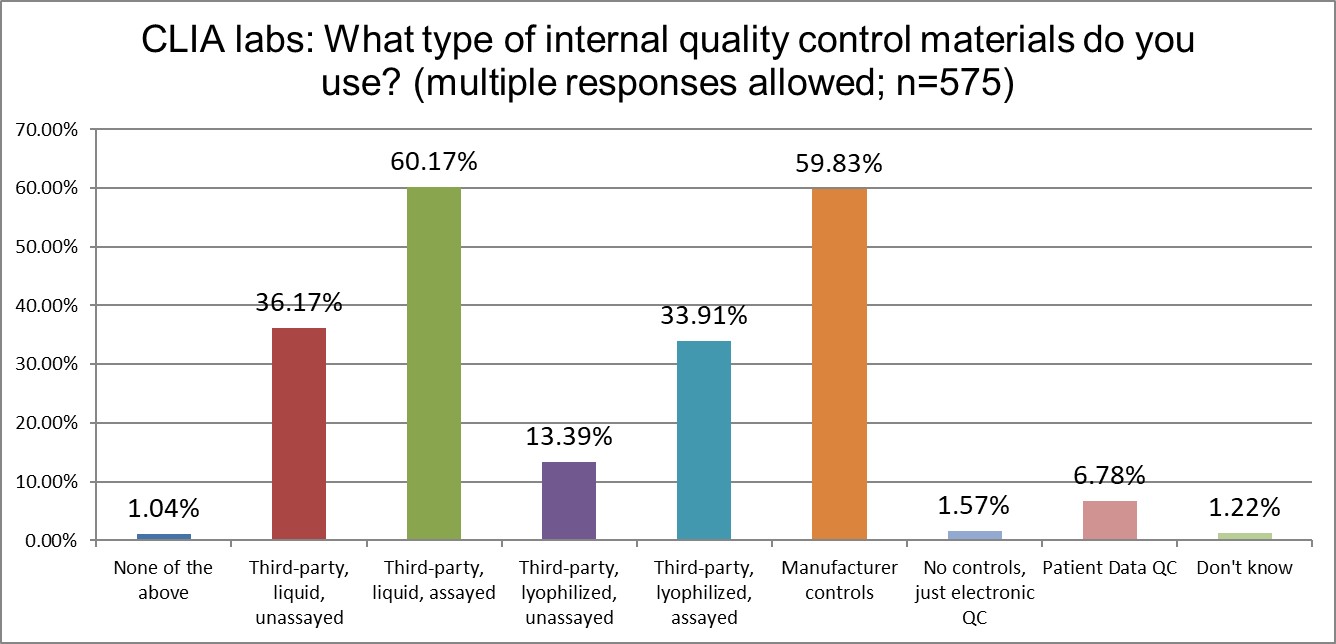
Using manufacturer controls is extremely common across both groups of labs. However, CLIA labs use 3rd party assayed liquid controls just slightly more than manufacturer controls. CLIA labs seem to favor liquid controls more, while ISO labs tilt slightly more toward lyophilized controls.
One other minor difference is the popularity of patient data QC. ISO labs like it (about 11%) more than CLIA labs (about 7%). Neither standard really demonstrates encouragement of the use of these techniques.
Just how much QC is patient-based?
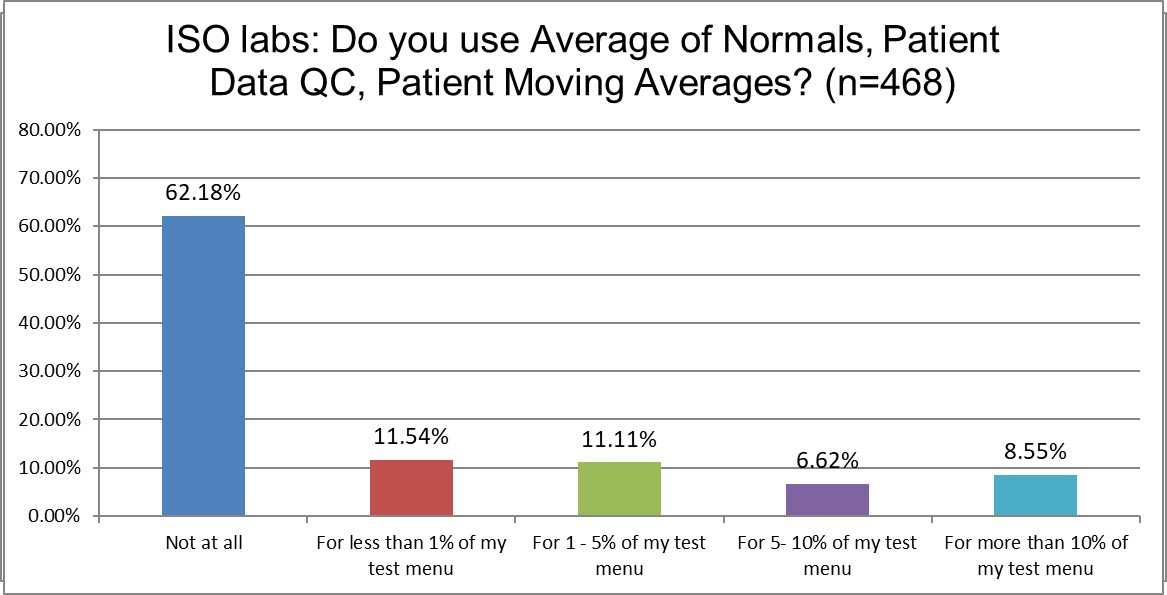
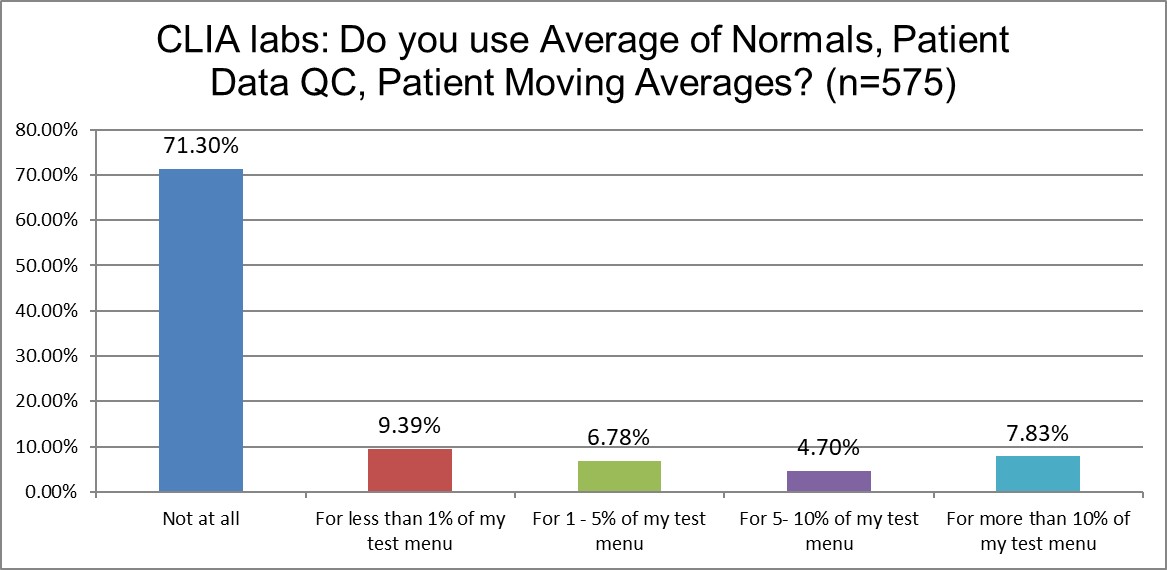
There are not big differences in the use - or non-use - of patient data QC techniques in either group of labs. CLIA labs don't use patient data QC at all at a higher rate than ISO labs. The use of the technique on a significant number of the test menu (>10%) is basically the same among both lab groups (around 8%).
PBRTQC remains a bespoke technique, rarely used, and not at a scale where it can provide robust solutions to laboratory QC challenges. Neither ISO nor CLIA are apparently making the technique more attractive to labs.
The Frequency of Running Controls
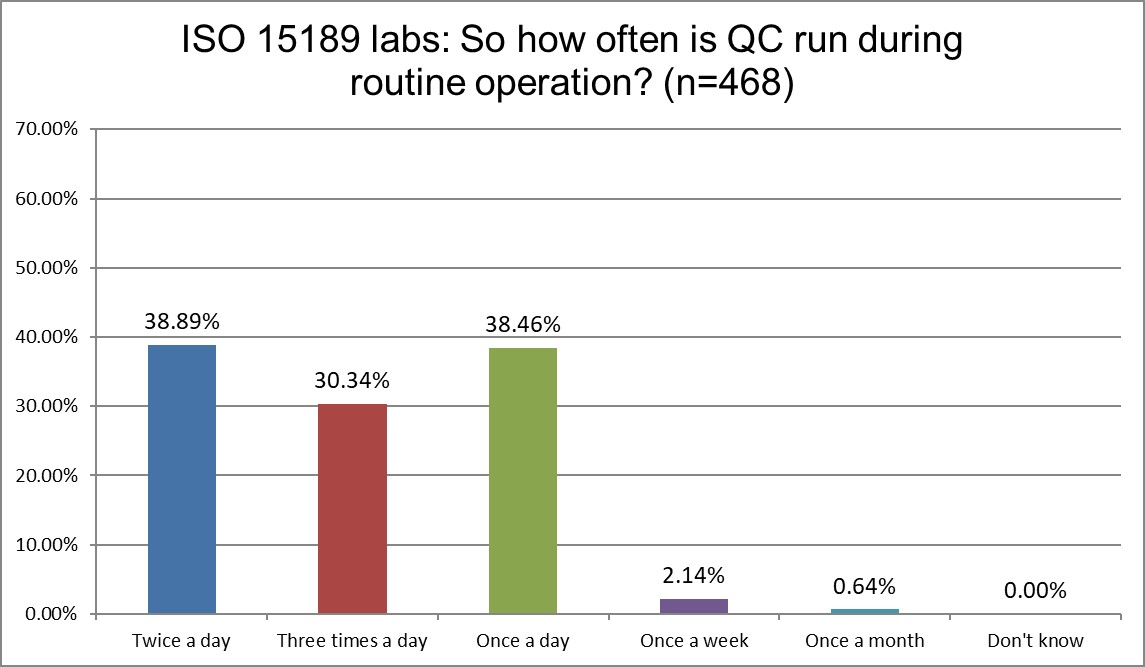
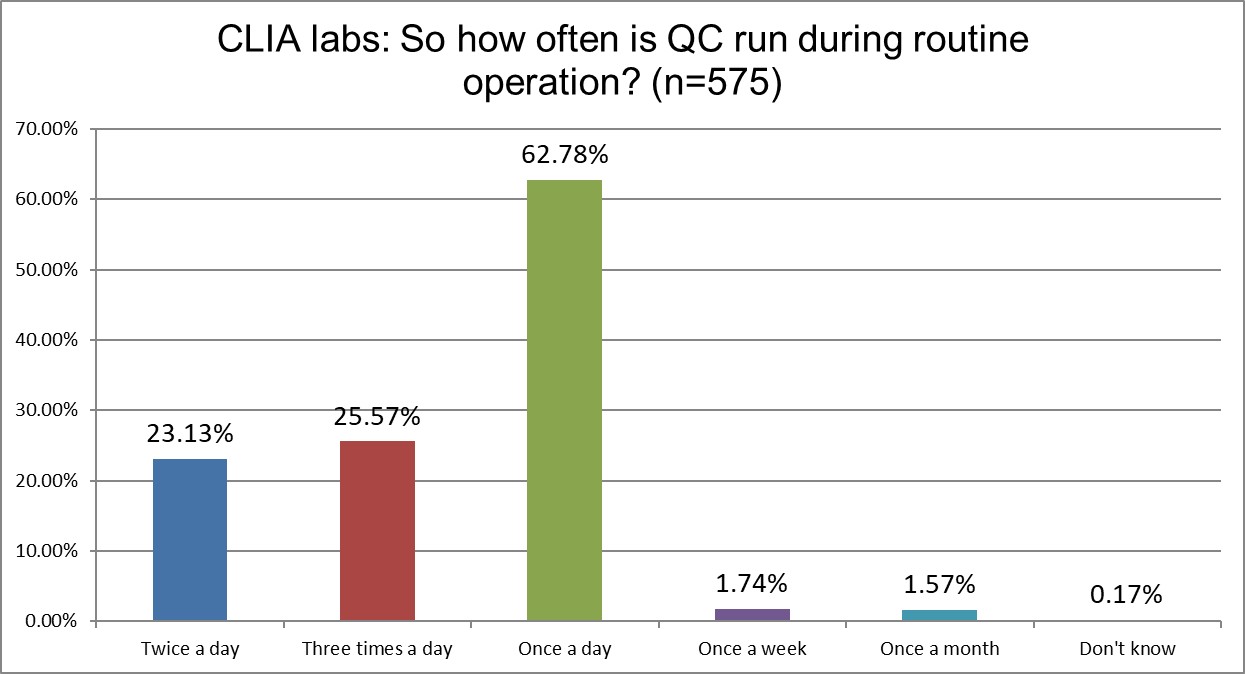
Here are the first real differences between ISO and CLIA labs. CLIA labs are overwhelmingly once a day QC users, while ISO labs are more balanced across once,twice or three times a day. Here's where the specificity of CLIA meets the vagueness of ISO. ISO doesn't give a detailed recommendation for QC frequency. CLIA demands a minimum of once a day. When CLIA makes a minimum requirement, labs rarely go above it. The minimum transforms into a maximum.
Who has more trouble when troubleshooting? ISO or CLIA?
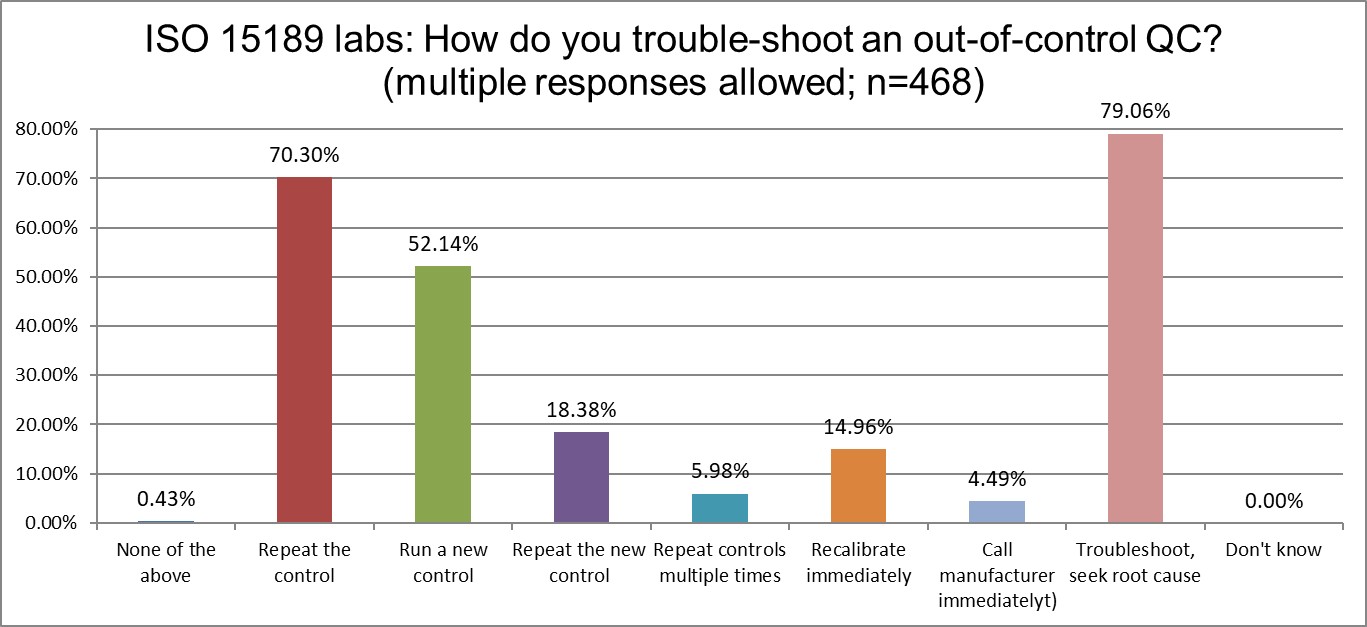
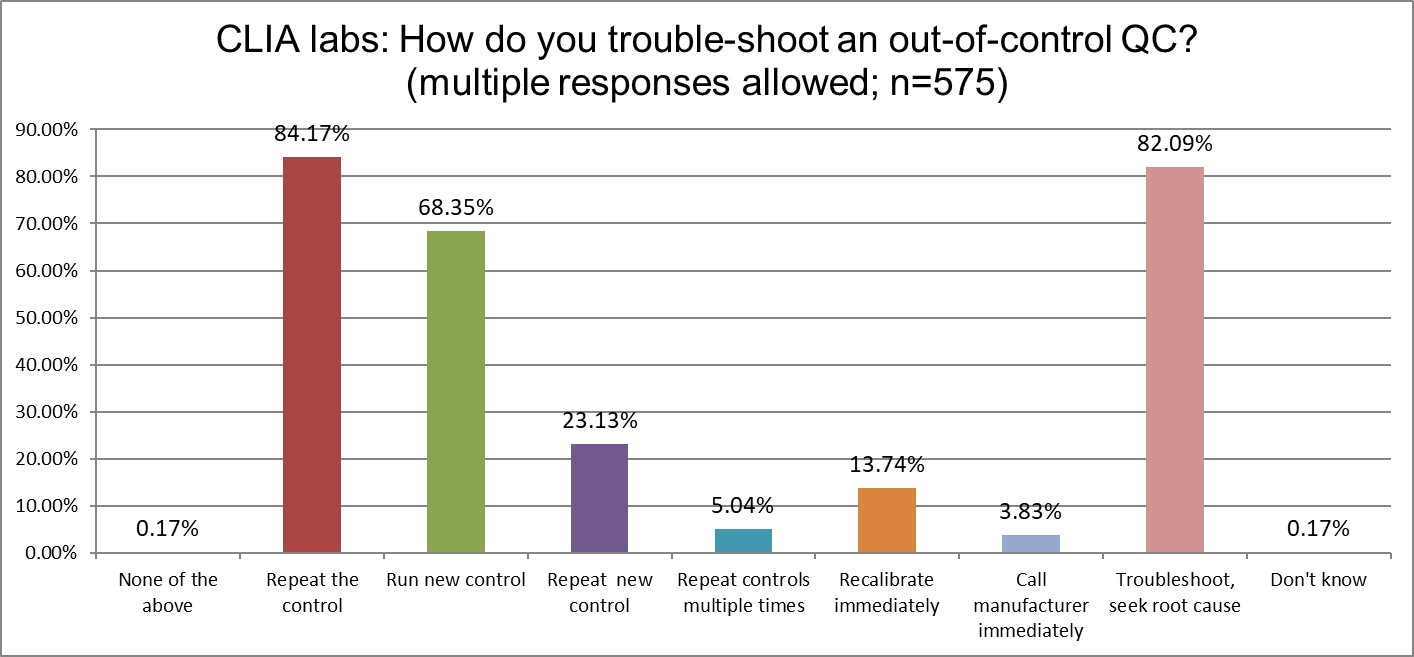
Both lab groups like to properly troubleshoot and seek root cause. However, both misunderstand what "proper" really means. CLIA labs love to repeat controls (84%), but ISO labs are almos equally enamoured of the practice (70%). CLIA labs also favor running new controls more than ISO labs (68% vs 52%), repeating those controls again (23% vs 18%), but the groups are about equally likely to just keep repeating and repeating (about 5%). On the whole, CLIA labs are indulging more in bad troubleshooting habits than ISO labs. But this is a relative comparison. Both groups are still quite significantly wasting time, effort, and resources on bad QC habits. Neither standard provides the cure.
Who's out of control more often? CLIA or ISO labs?
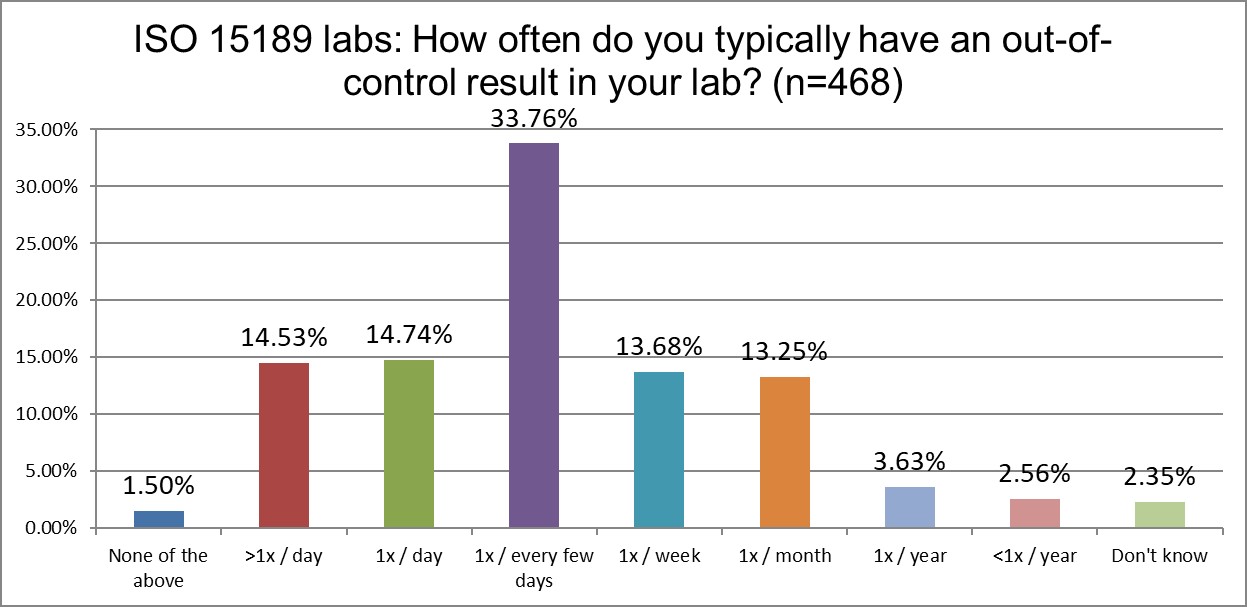
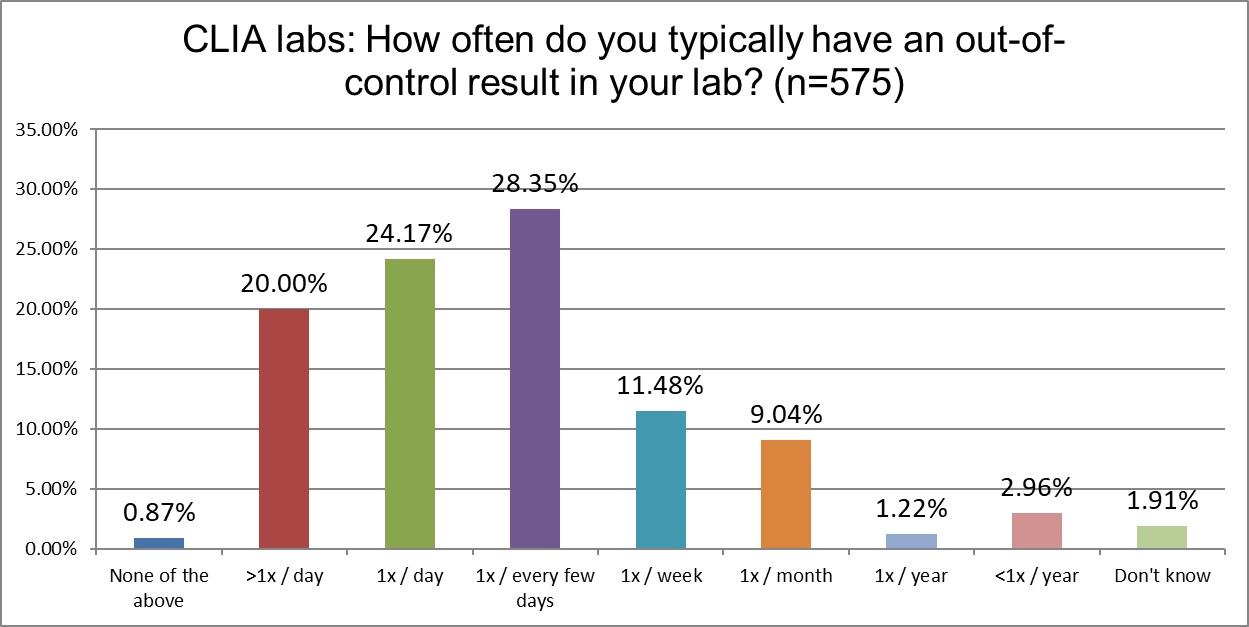
Another significant difference emerges when we examine the frequency of out-of-control events. CLIA labs, just above 44% of labs are out of control every day if not multiple times per day. For ISO labs, that's only a little more than 29%. Interesting, because, as we saw earlier, ISO labs are running QC more often than CLIA labs. We saw earlier that CLIA labs use 2 SD limits slightly more than ISO labs, but that doesn't quite explain this difference. CLIA labs also use their own mean and SD at a higher rate than ISO labs, which could further contribute to more out-of-control events. Using the 1:2s rule, but with an SD that's not your own, will generate a lower rate of false (or true) rejections.
How do labs re-test?
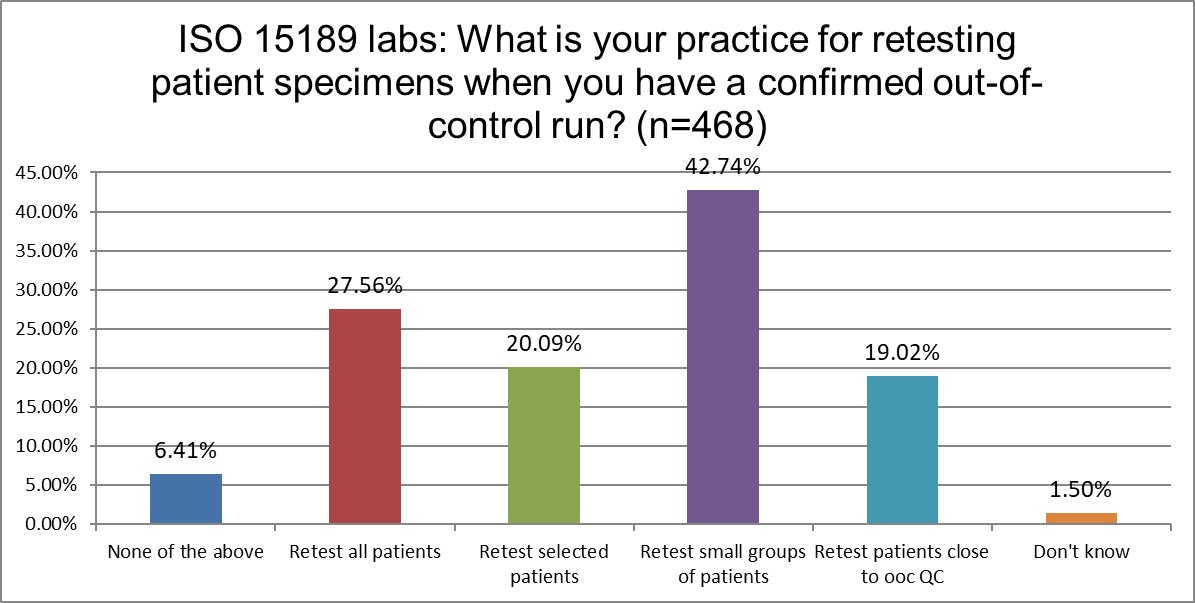
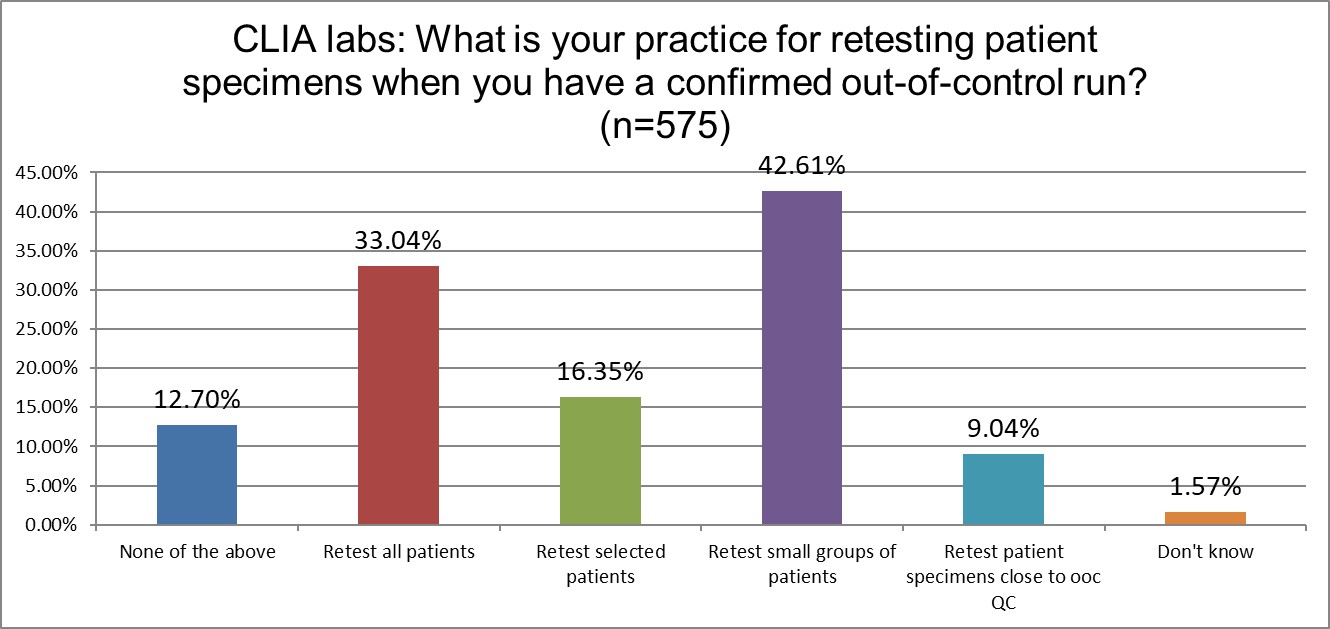
When it comes to repeating patients, there is not a large difference between ISO and CLIA labs. The largest difference is that ISO labs have a stronger preference (19% vs 9%) to retest patients close to the out-of-control level.
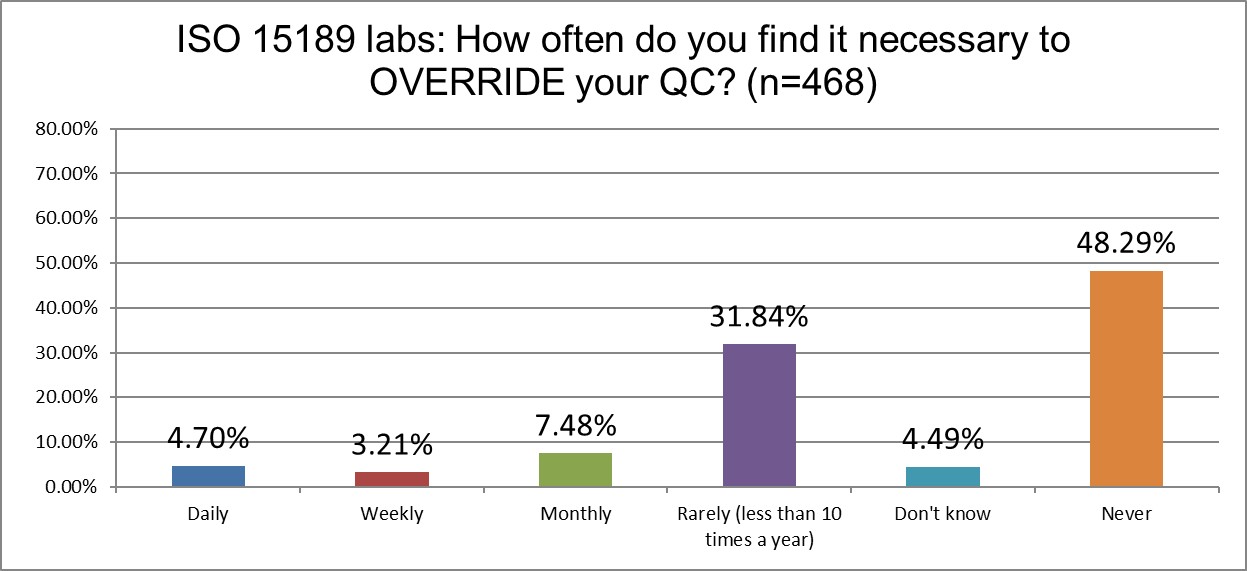
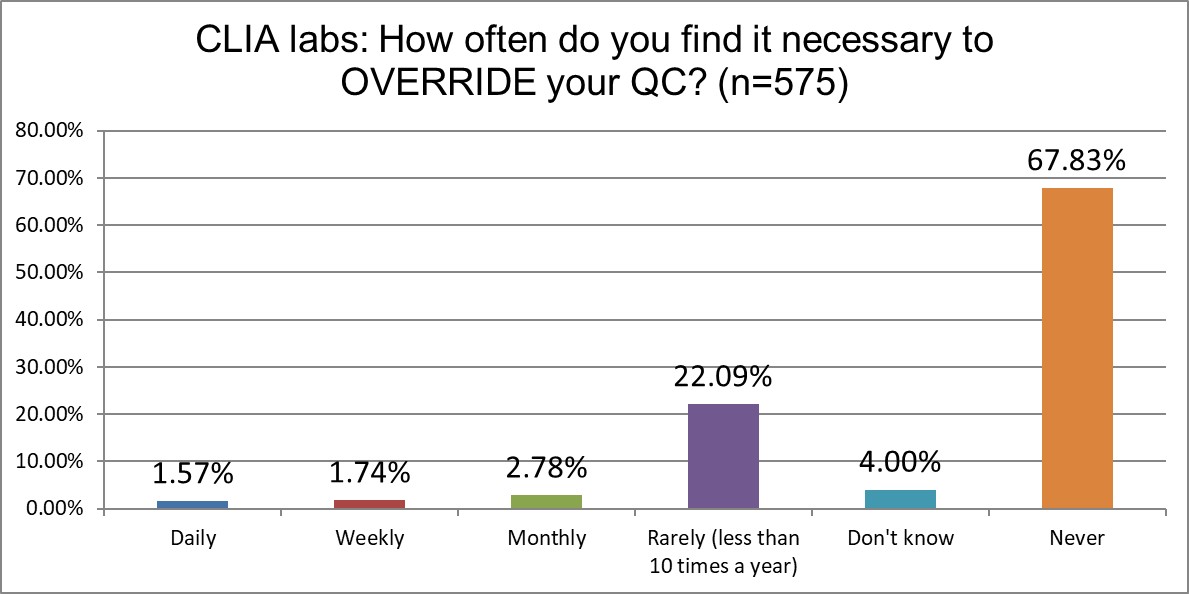
How likely are labs of different sizes to override their own QC?
ISO labs are significantly more likely to override their own QC than CLIA labs (about >15% vs 6%). CLIA labs are also significantly more likely to never override their QC to release results (nearly 68% vs about 48%).
Releasing patient results even when QC is out is a practice neither CLIA nor ISO would endorse, but neither are very specific about that.
Which labs include the uncertainty of bias correction in their measurement uncertainty calculations?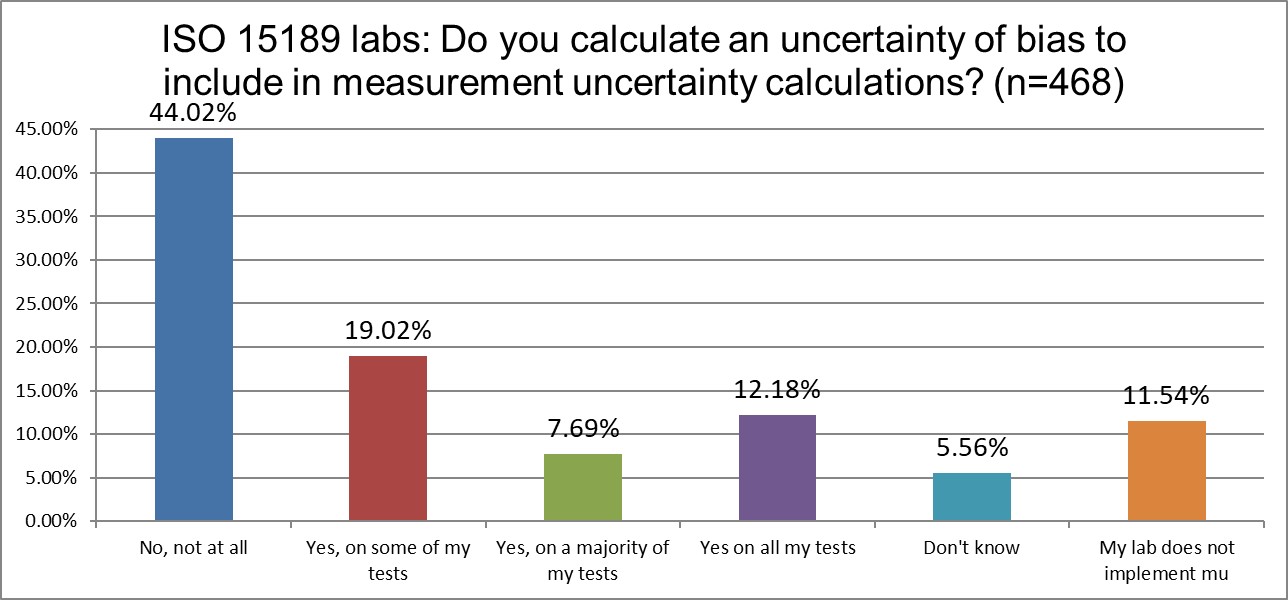
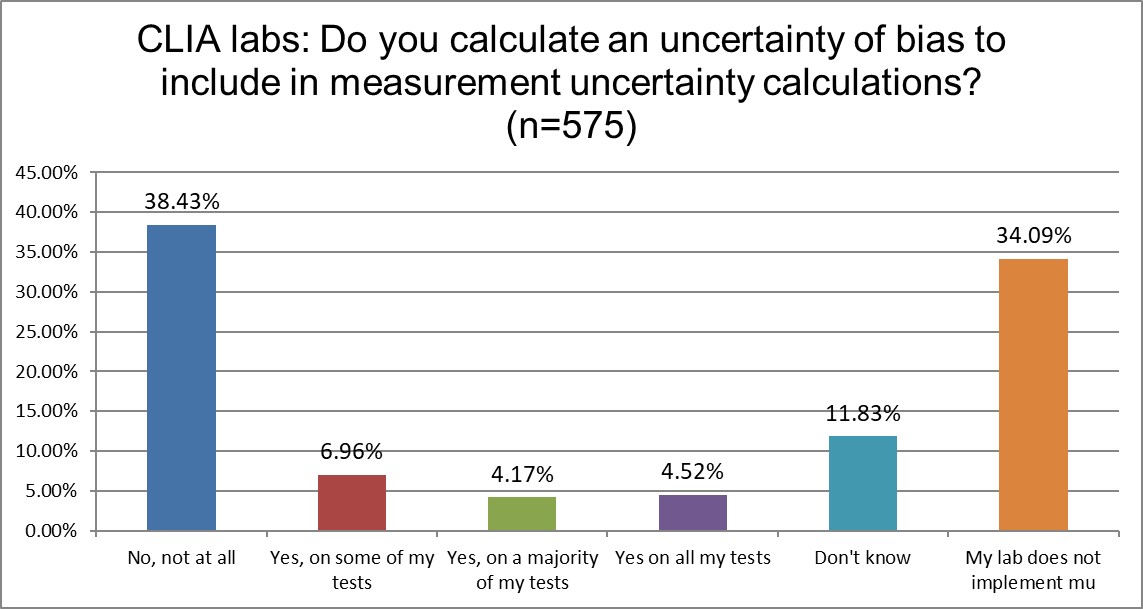
Given that ISO 15189 contains a mandate to calculate measurement uncertainty, while CLIA does not even mention the concept, we would expect to see greater differences in the reporting of measurement uncertainty. So it's not surprising that there are more CLIA labs that don't calculate mu than ISO labs (34% vs 11%). However, we might have expected a much higher percentage from the CLIA labs, and it's also surprising that so many labs accredited to ISO are violating their mandates. A high rate of the ISO labs are also avoiding the inclusion of correction of bias uncertainty. But this uncertainty of bias correction is just the latest fad in mu. The base mandate is to calculate mu, which is typically taken as the standard deviation, with nothing more.
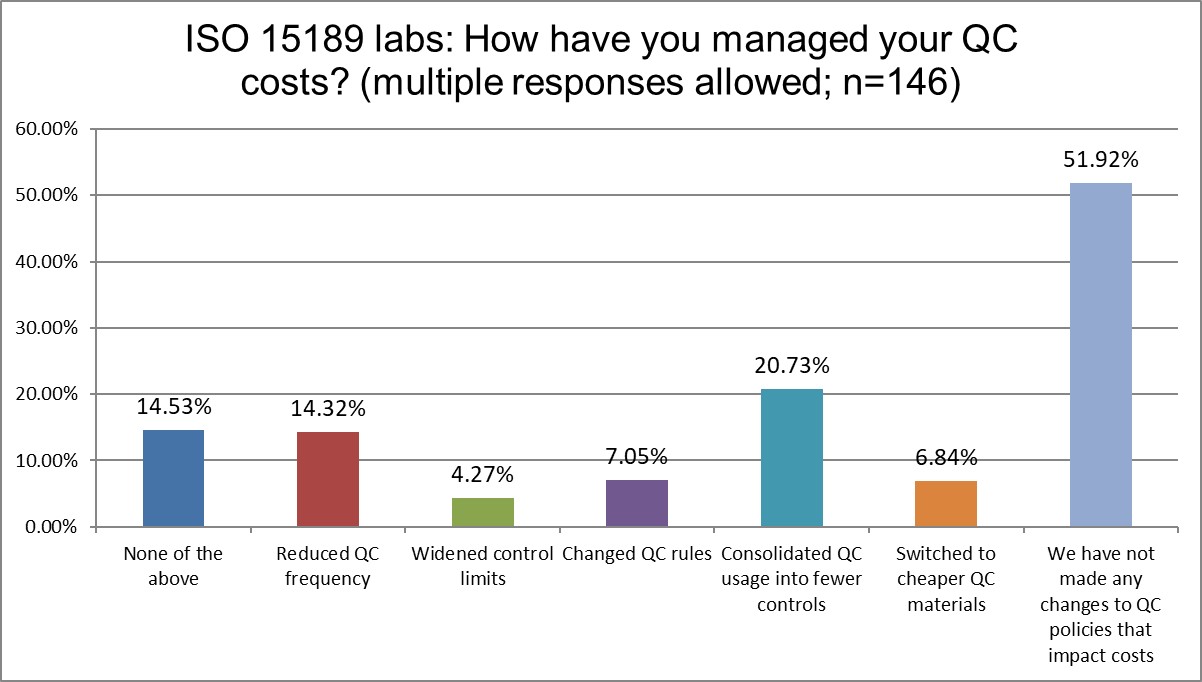
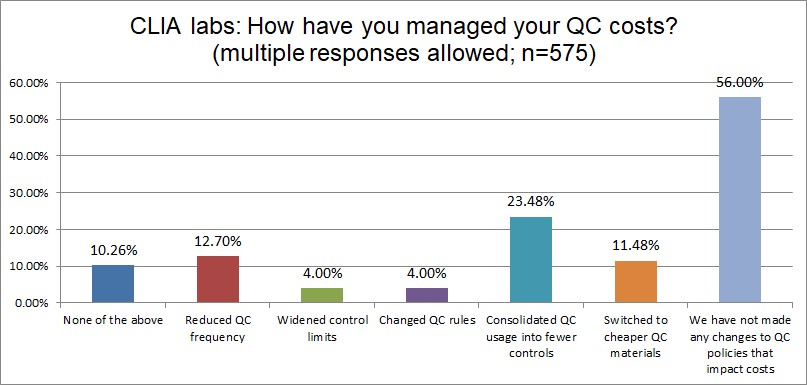
The Final Overview
A majority of labs both ISO and CLIA have done nothing to manage their QC costs. Otherwise, there is a lot of similarity
Conclusion
The similarities between CLIA and ISO labs demonstrate that neither standard is providing distinctive, productive advice when it comes to QC. If a laboratory is suffering from bad QC, adopting ISO 15189 or CAP accreditation is not a guaranteed cure. Labs seeking better QC practices need to look beyond the base regulations.