Basic QC Practices
The 2025 Great Global Survey: Public, Private and Reference labs
In 2025, the Westgard Great Global Survey assessed the state of QC. Among public, private, and high volume reference laboratories, do QC practices differ?
The 2025 Global QC Survey Results: Public, Private, and High Volume Reference Labs
Sten Westgard, MS
December 2025
[This survey was completed with the support and partnership of Thermo Fisher MAS controls.]
In 2025, have QC practices around the world improved or declined?
We surveyed laboratories in 2017 and 2021 about their quality control practices. We did it again in 2025.
We got over 1,280 complete, qualified responses, which break down as follows:
- Africa 118 responses
- Asia 289 responses (note that we include everywhere from India to Australia within this group)
- Europe 143 responses
- Latin and South America 114 responses
- Middle-east 146 responses
- United States and Puerto Rico 440 responses
Asia: 2025 Great Global QC Survey Results: Asia Breakout - Westgard QC
Europe: The 2025 Great Global QC Survey: Europe in isolation - Westgard QC
Middle-East: 2025 Great Global QC Survey Results: Middle East - Westgard QC
Latin and South America: 2025 Great Global QC Survey Results: South and Latin America - Westgard QC
Africa: 2025 Great Global QC Survey Results: Africa - Westgard QC
USA: https://westgard.com/qc-applications/basic-qc-practices/2025-qc-survey-usa.html
All of it together: https://westgard.com/qc-applications/basic-qc-practices/2025-global-qc-survey.html
While we've completed regional analyses, we decided to look deeper at the differences between different types laboratories.
- public laboratories 423 responses
- private laboratories 216 responses
- high volume reference laboratories 65 reponses
This last group is small, because it's made up of a more focused segment, reference and independent labs with volumes of 1MM or higher.
Please note, you can click on these graphs and expand them to a larger size. Here they are shrunk down to help make the comparisons easier to visualize.
The QC Set Up
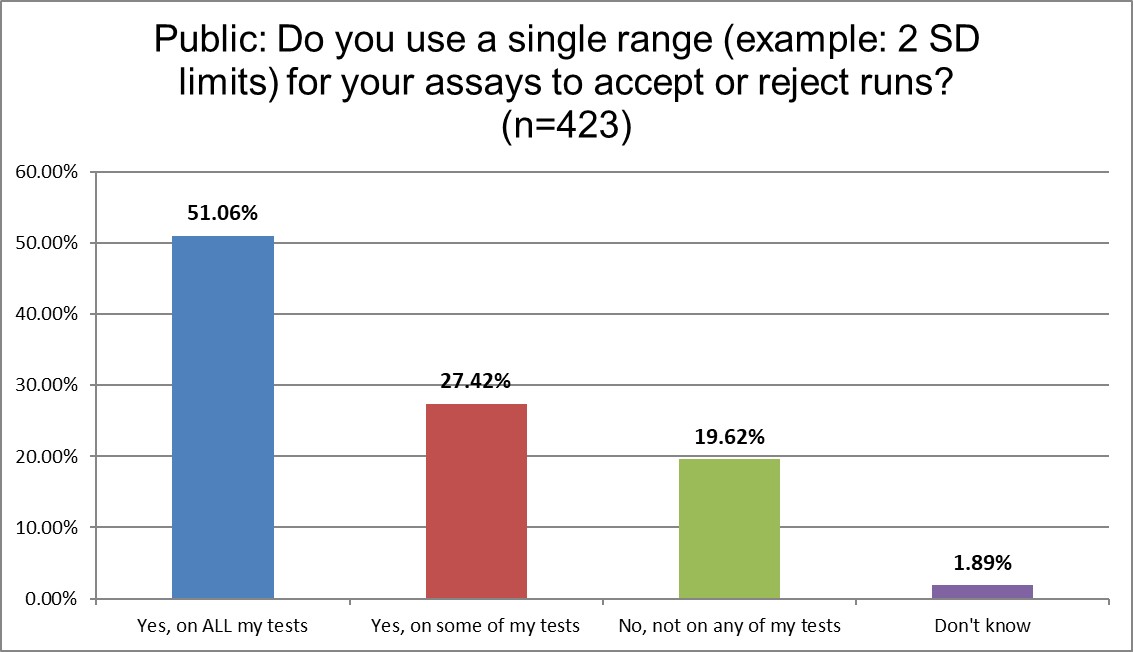
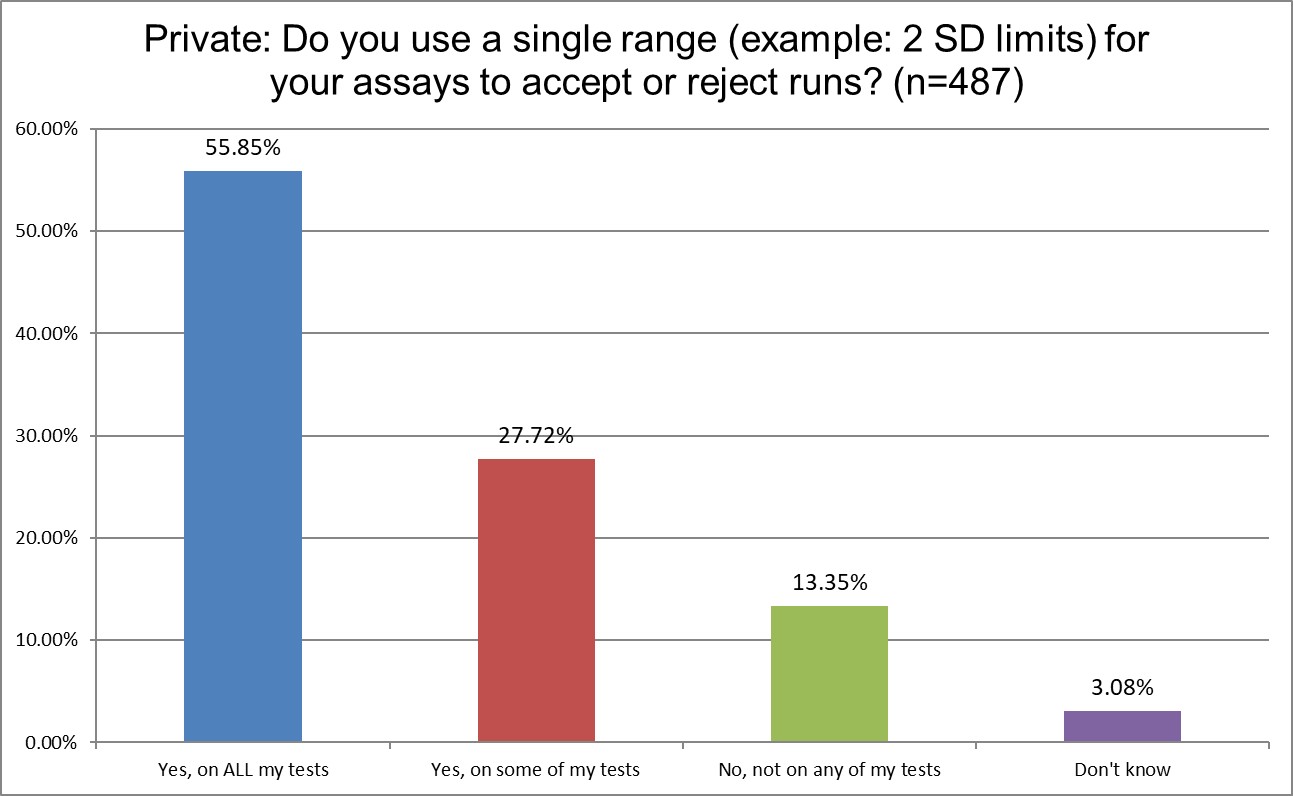
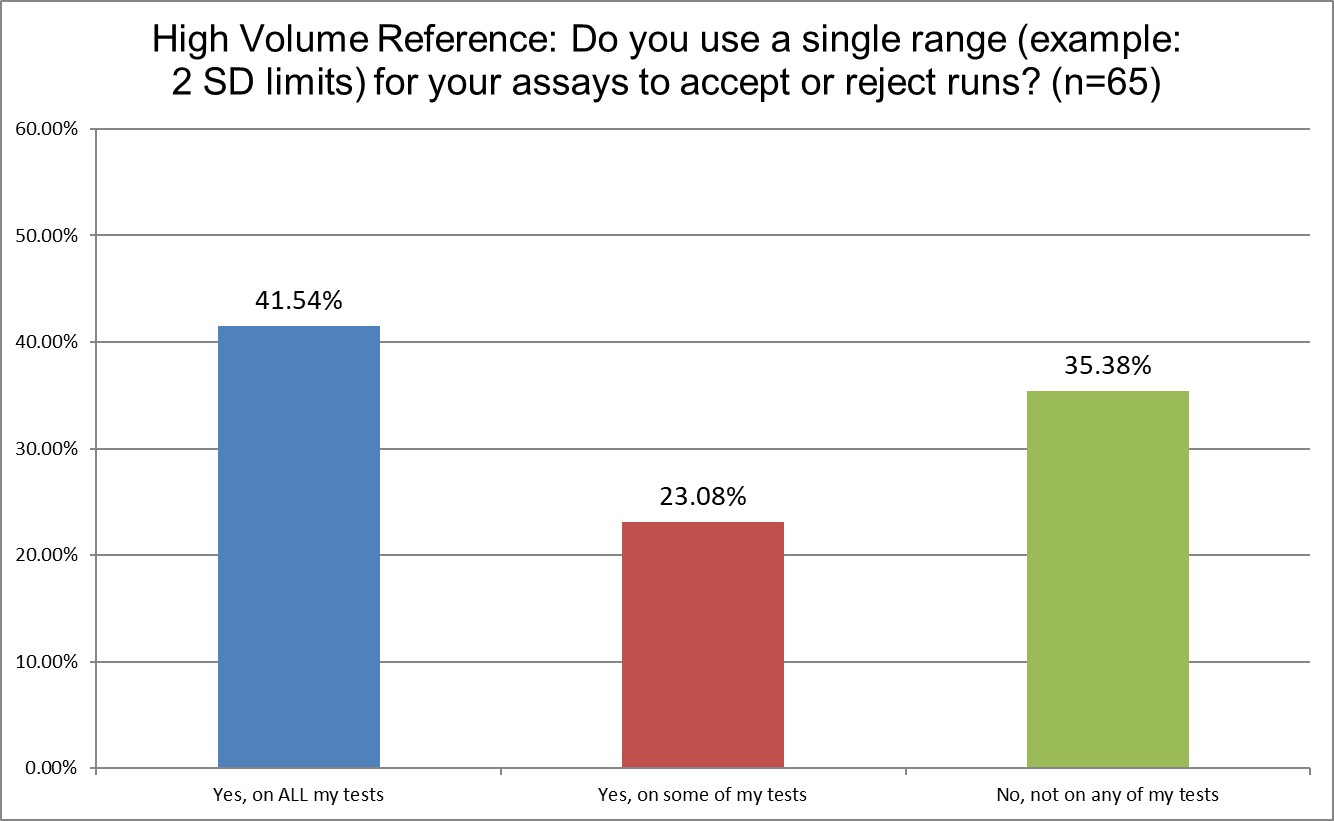
The use of 2 SD in hematology and chemistry differs considerably. 78.4% of Public labs use 2 SD on some or all of their tests. Private labs use them more, 83.6%. Only 64.6% of High volume reference labs (hereafter shortened to Hi Ref) are using 2 SD on some or all of their tests. Almost a 20% gap between Private and Hi Ref labs!
Warning, Rejection, or Both?
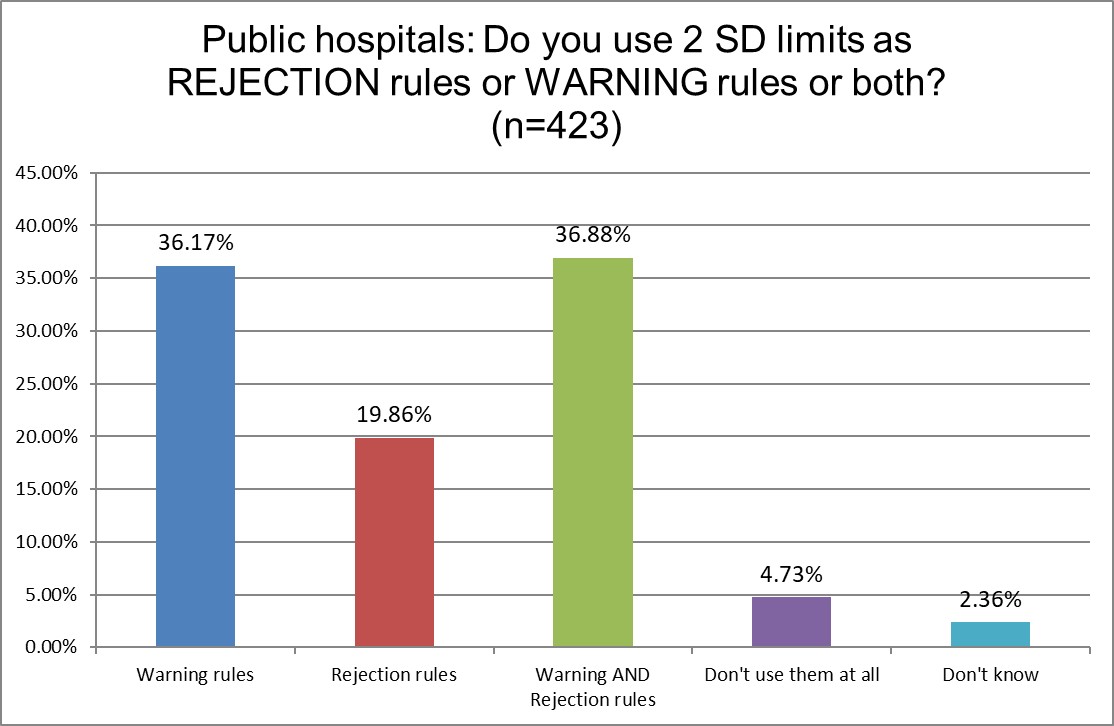
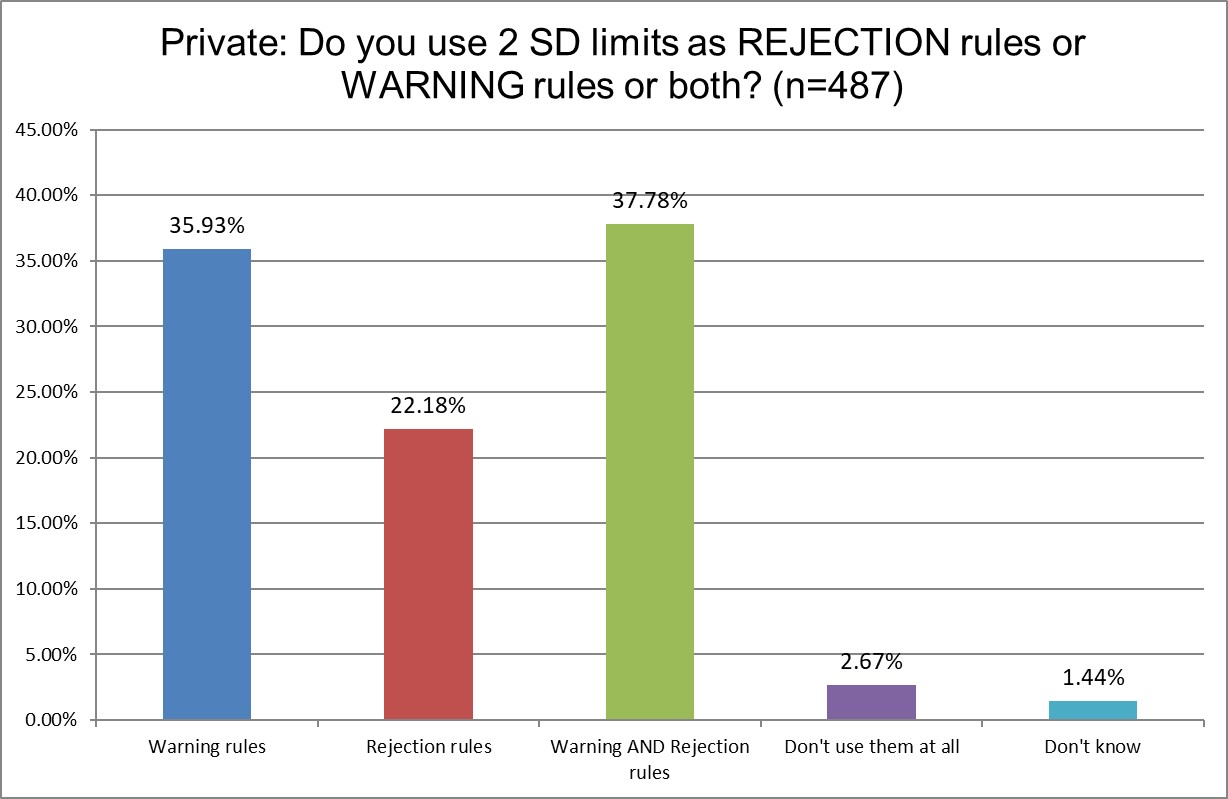
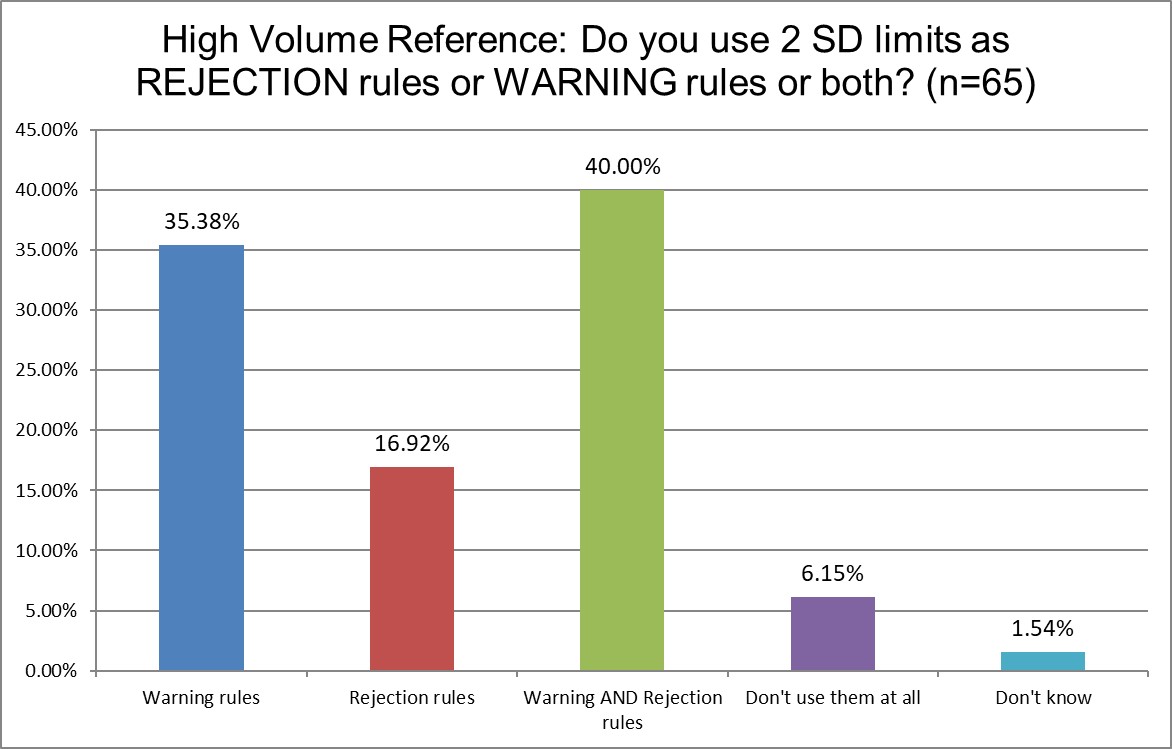
There are more similarities this time. But we do see that Hi Ref laboratories are the least likely to use 2 SD limits for rejection, and most likely to use 2 SD for both warning and rejection limits. Hi Ref labs are also much more likely to not use 2 SD limits at all - as we saw in the previous question responses. What is it that these labs know that public and private labs don't? Perhaps having such high volume has shown them the cost of 2 SD high false rejections is untenable.
Who uses "Westgard Rules" the most? the least?
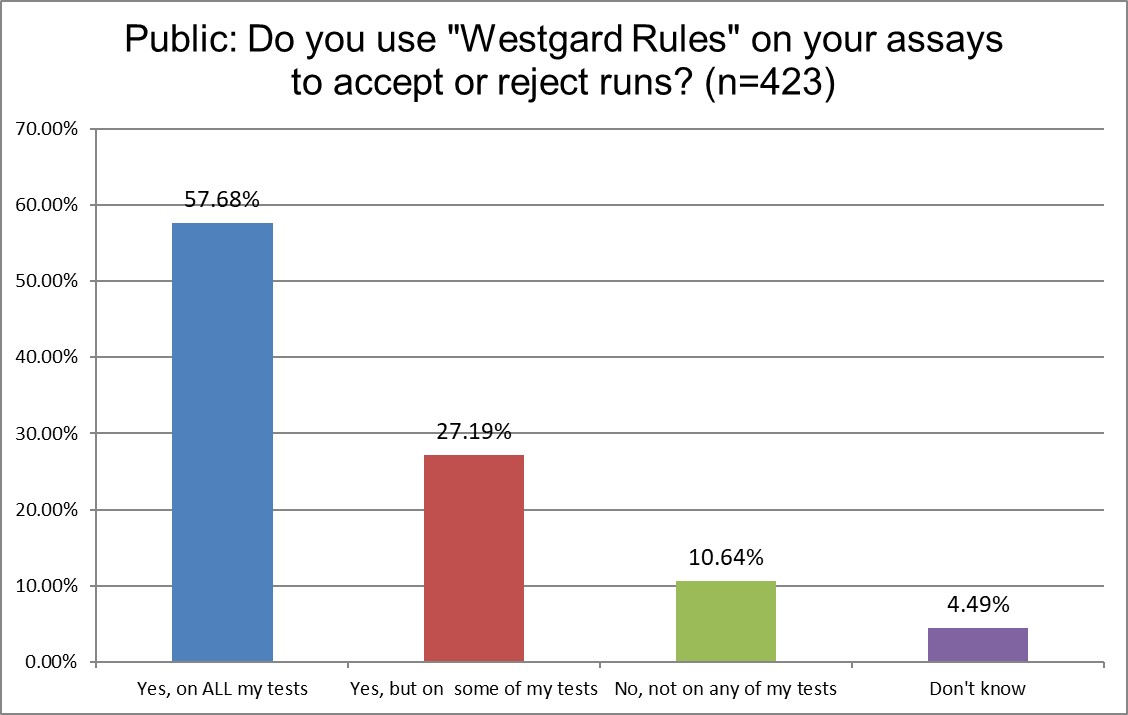
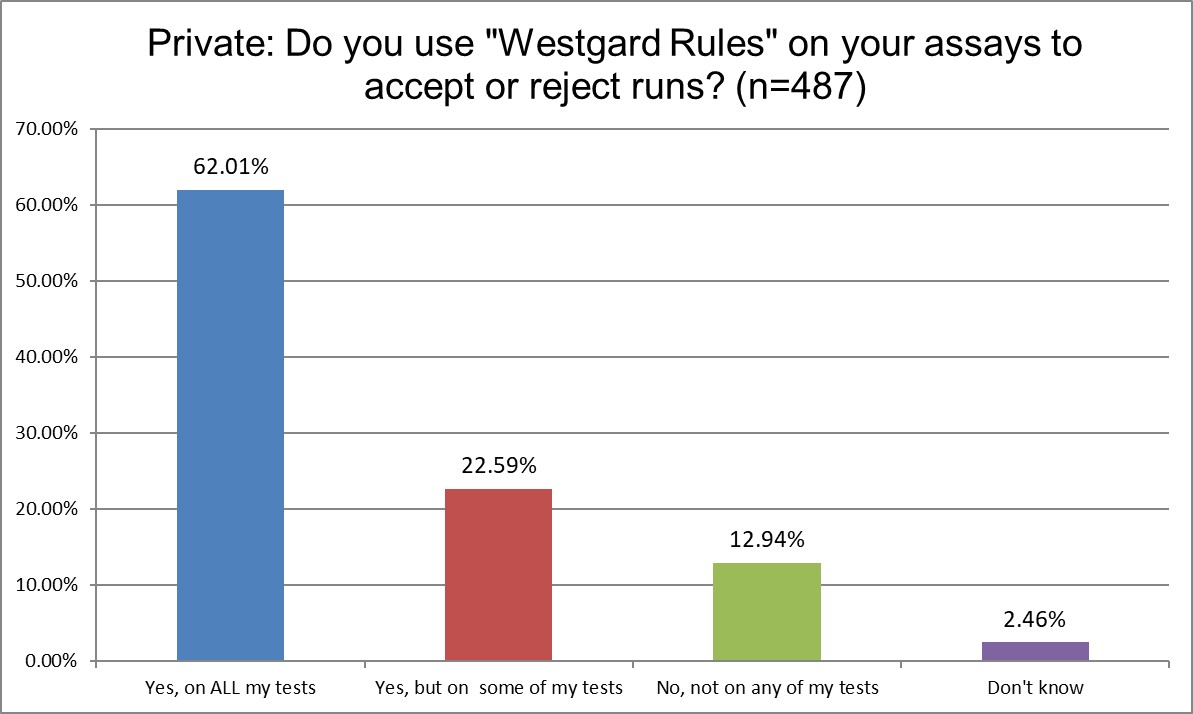
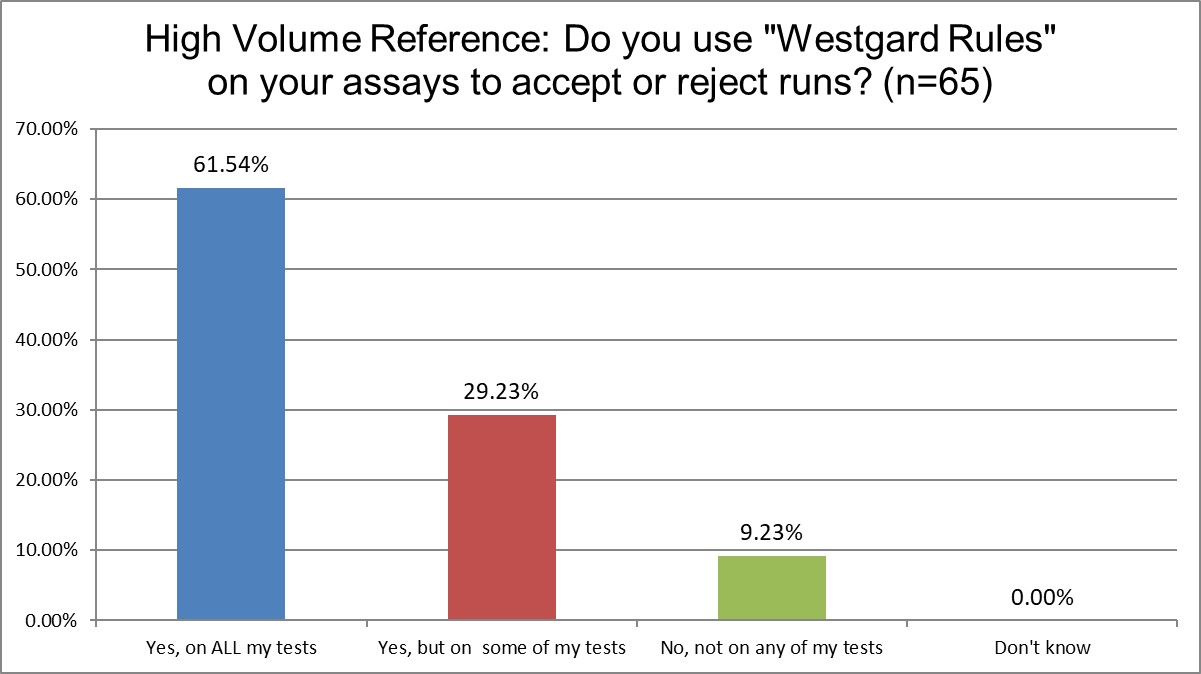
Public and Private labs are almost the same when it comes to using "Westgard Rules." 84.9% of Public labs use them on some or all of their tests, vs. 84.6% of Private labs, probably a statistically insignificant difference. Hi Ref labs, however, use them significantly more, 90.8% are using the Westgard Rules on some or all of their tests. Since Hi Ref labs are using fewer 2 SD limits, it makes sense that they're using more Westgard Rules.
Are mean and SD choices influenced by laboratory type?
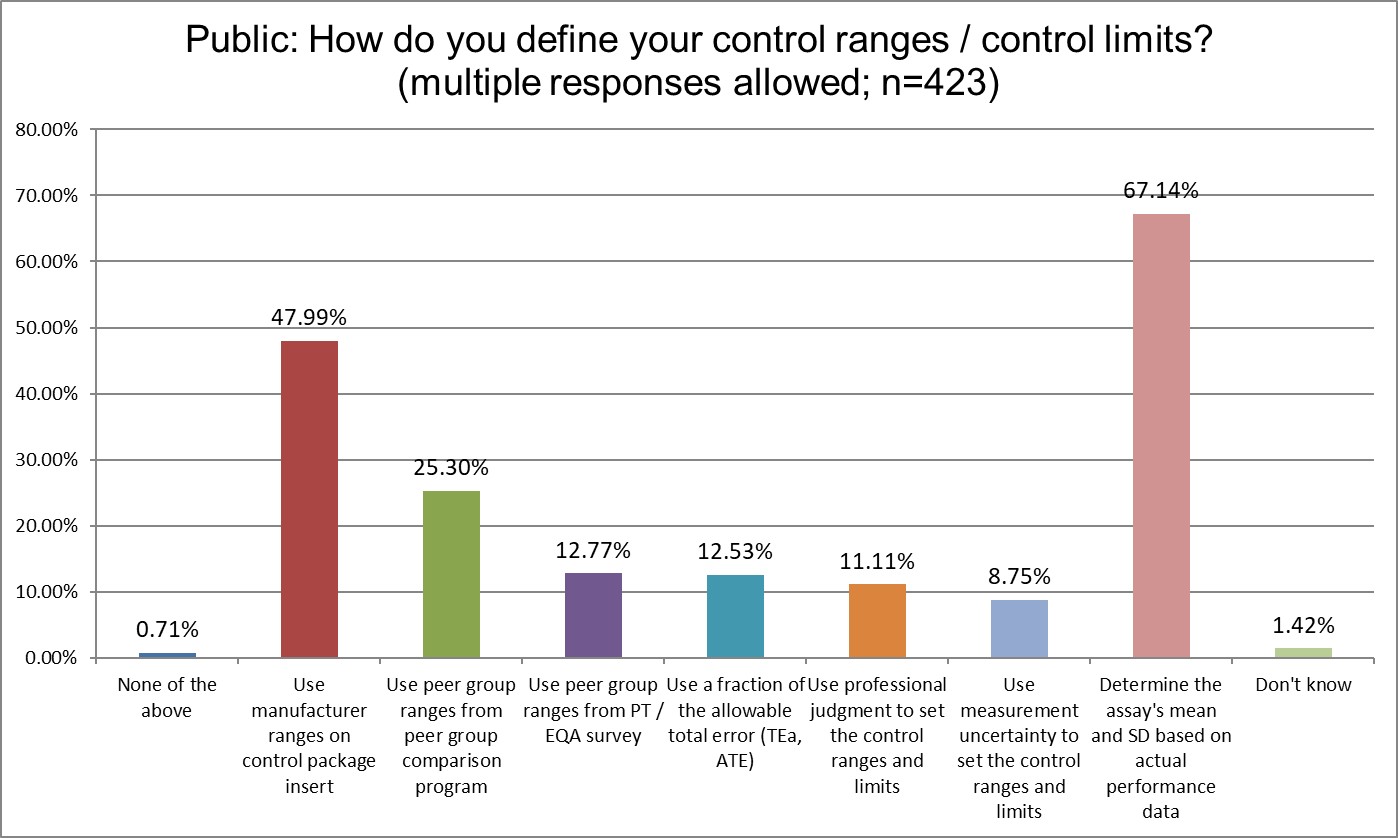
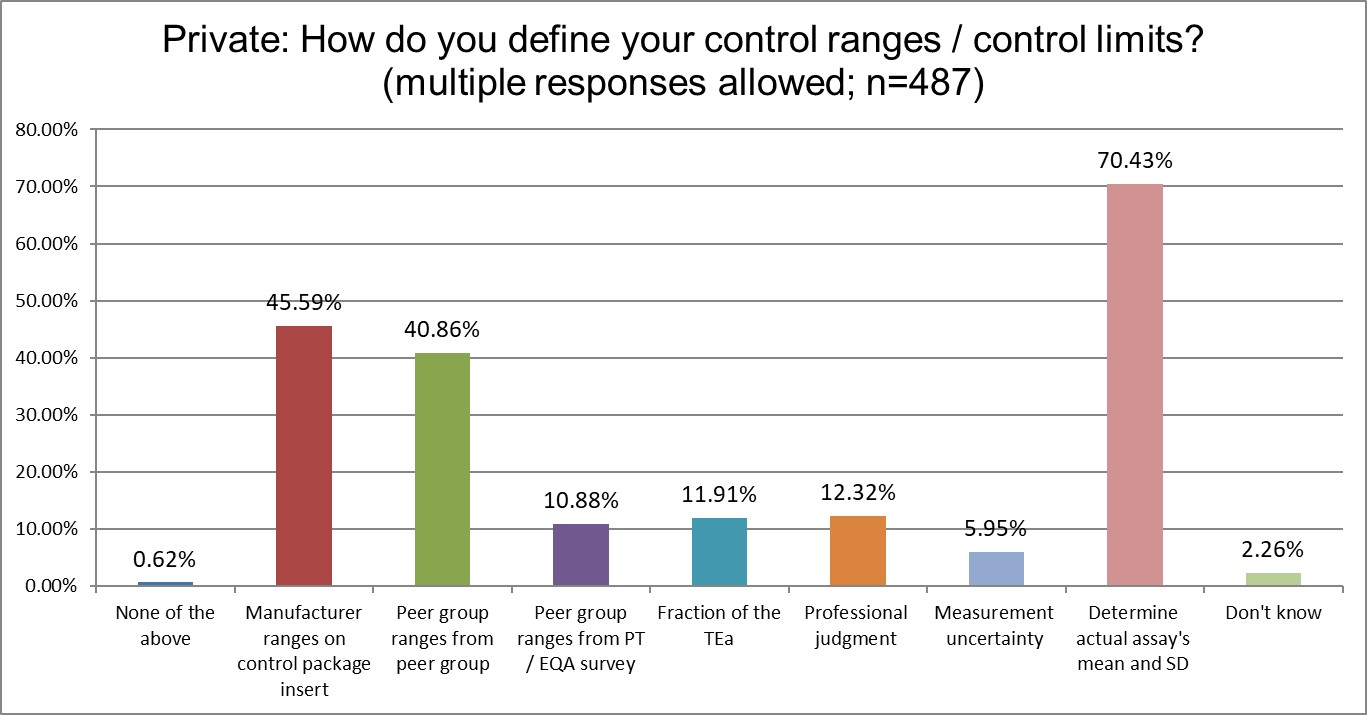
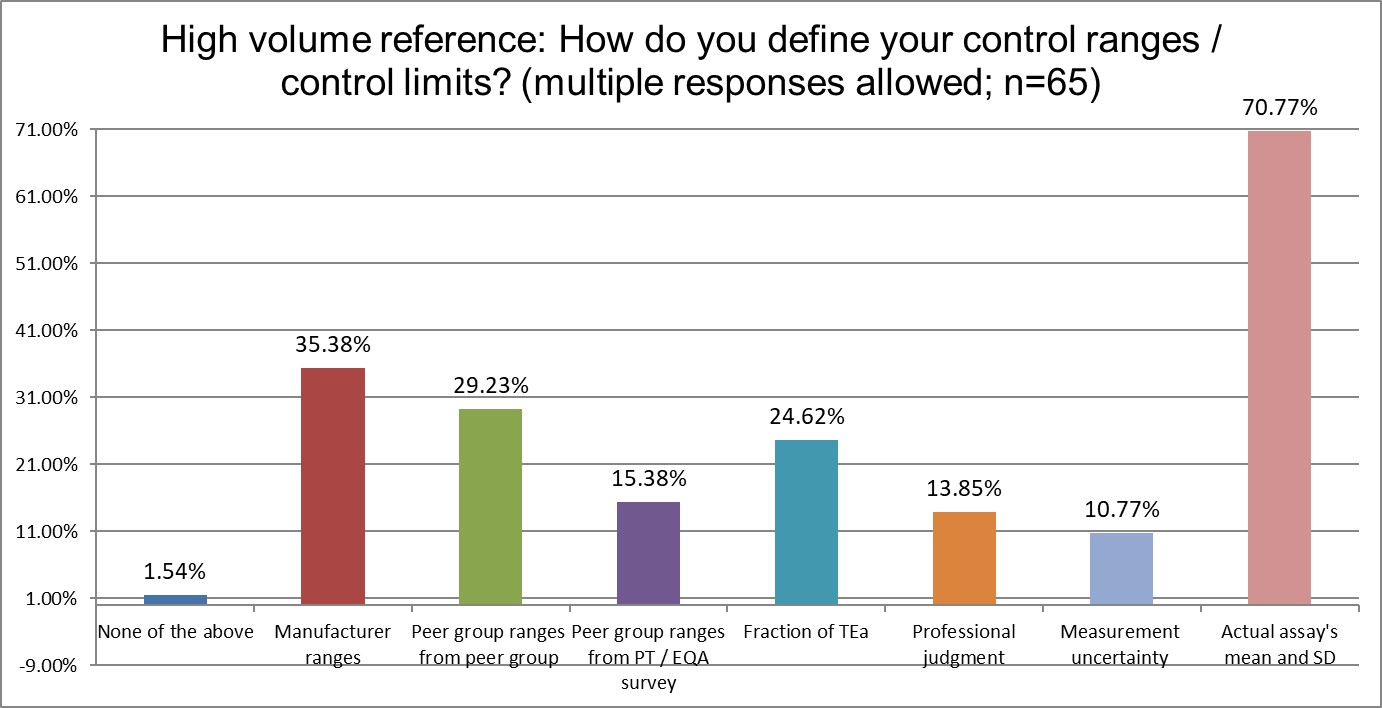
There are several differences between the groups. Public and Private labs are significantly more likely to use manufacturer ranges than Hi Ref labs. Private labs are significantly more likely to use the Peer group ranges from peer group programs for their ranges. Hi Ref labs are significantly more likely to use a fraction of the TEa to set ranges, which an unusual approach, and a unique finding. Overall, the use of the actual mean and actual SD of the method is the same proportion by each group.
What type of controls are more popular in Public, Private and Hi Ref labs?
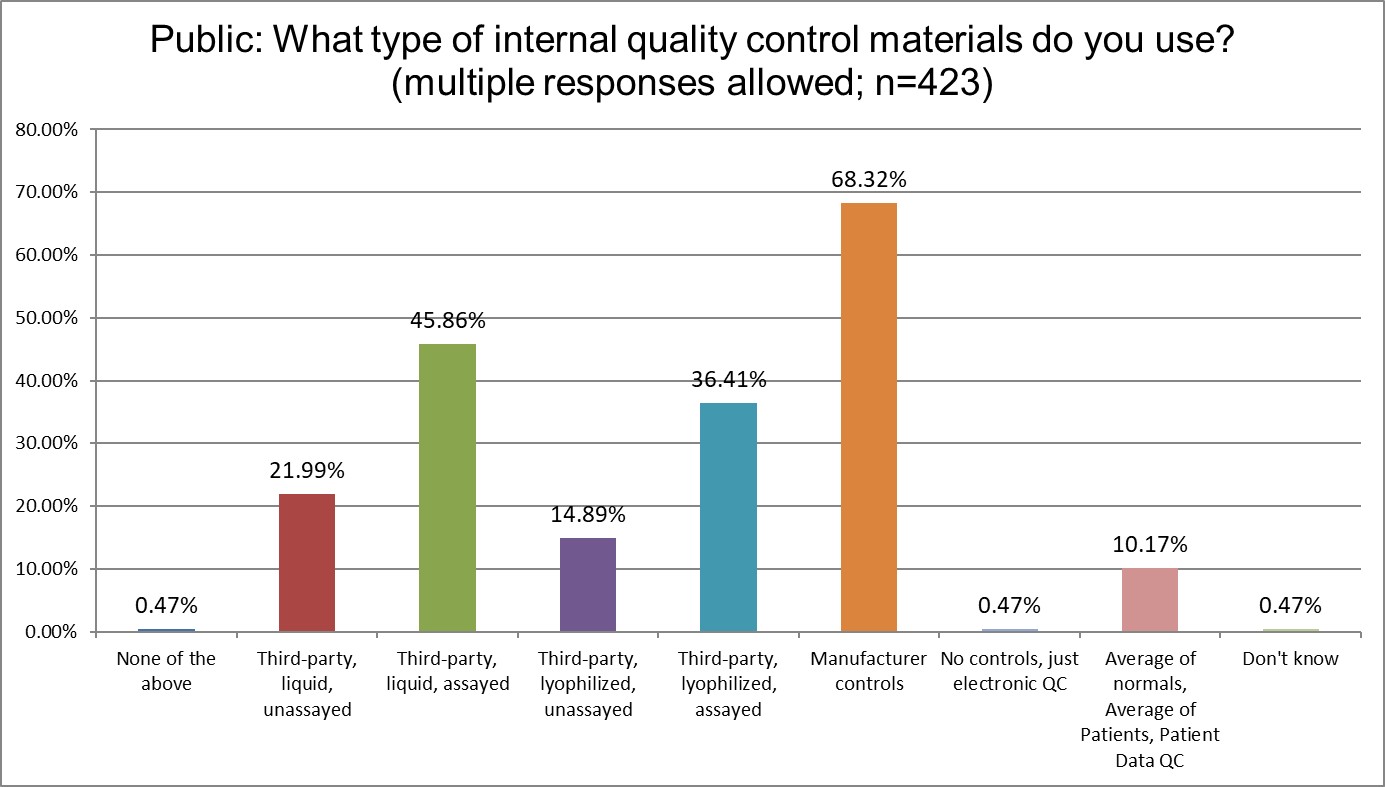
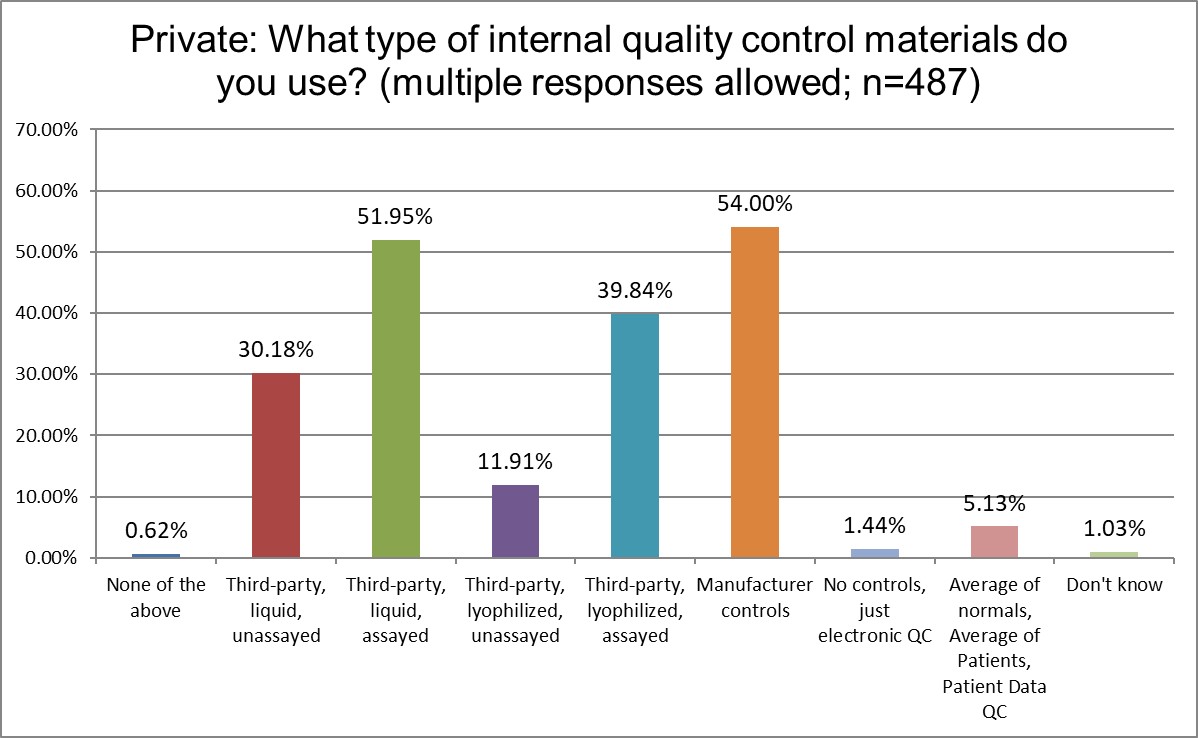
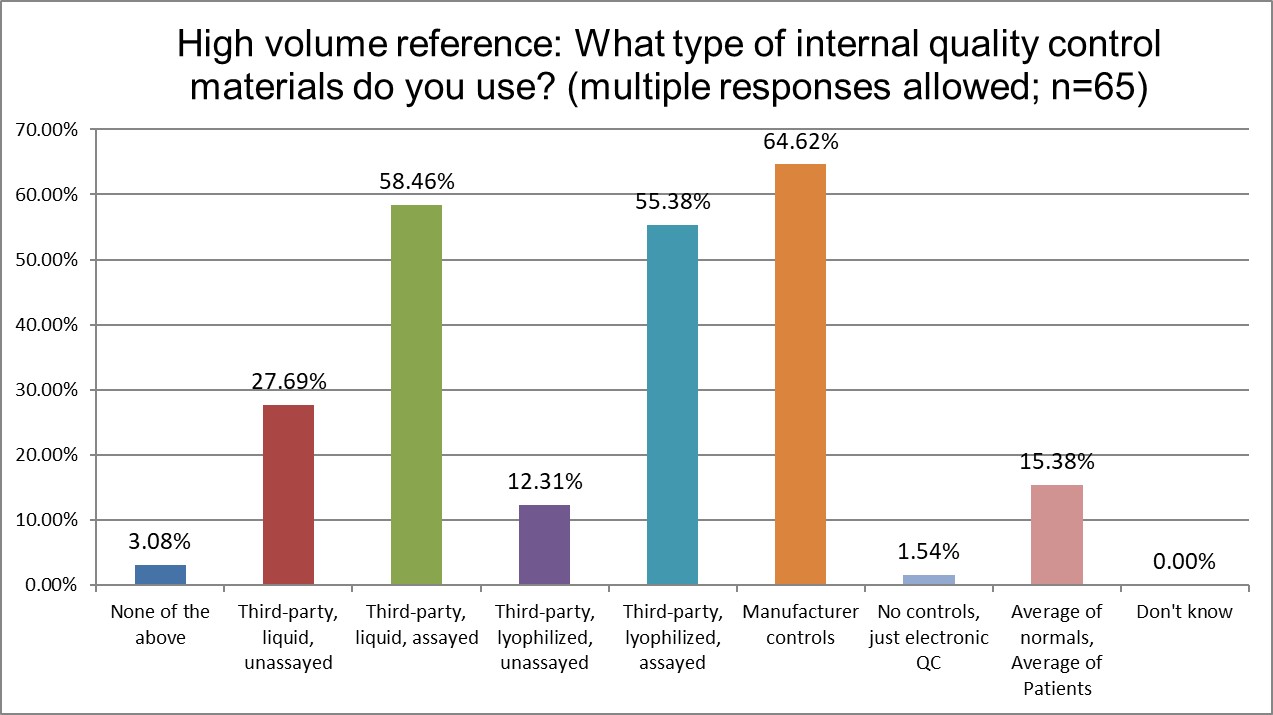
Manufacturer controls are most popular for all lab types, but they are most most popular for Public labs. More Hi Ref labs use the 3rd party lyophilyzed assayed controls and 3rd party liquid assayed controls than Public or Private labs. Patient-based QC (sometimes called PBRTQC) is significantly different in popularity: about 10% of Public labs, about 5% of Private labs, and about 15% of Hi Ref labs say they are using these techniques. Interesting, though, to see that despite all the hype, Patient based QC is really not very popular at all. There's been a lot of talk, but very little implementation.
Just how much QC is patient-based?
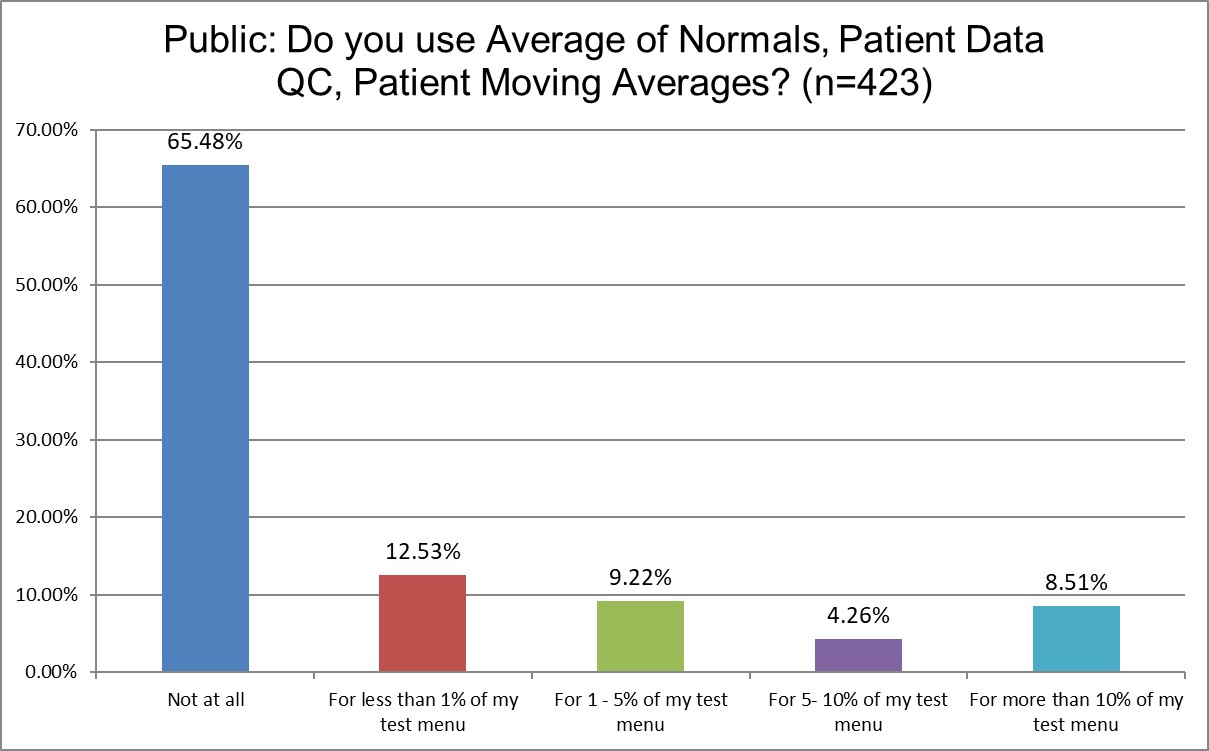
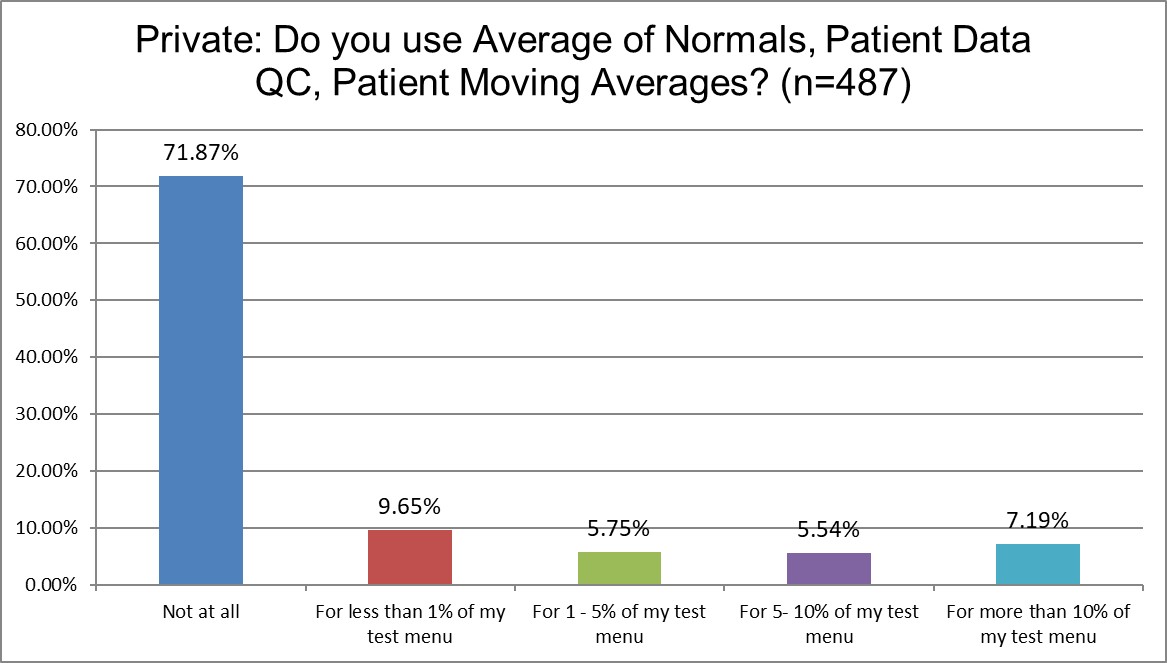
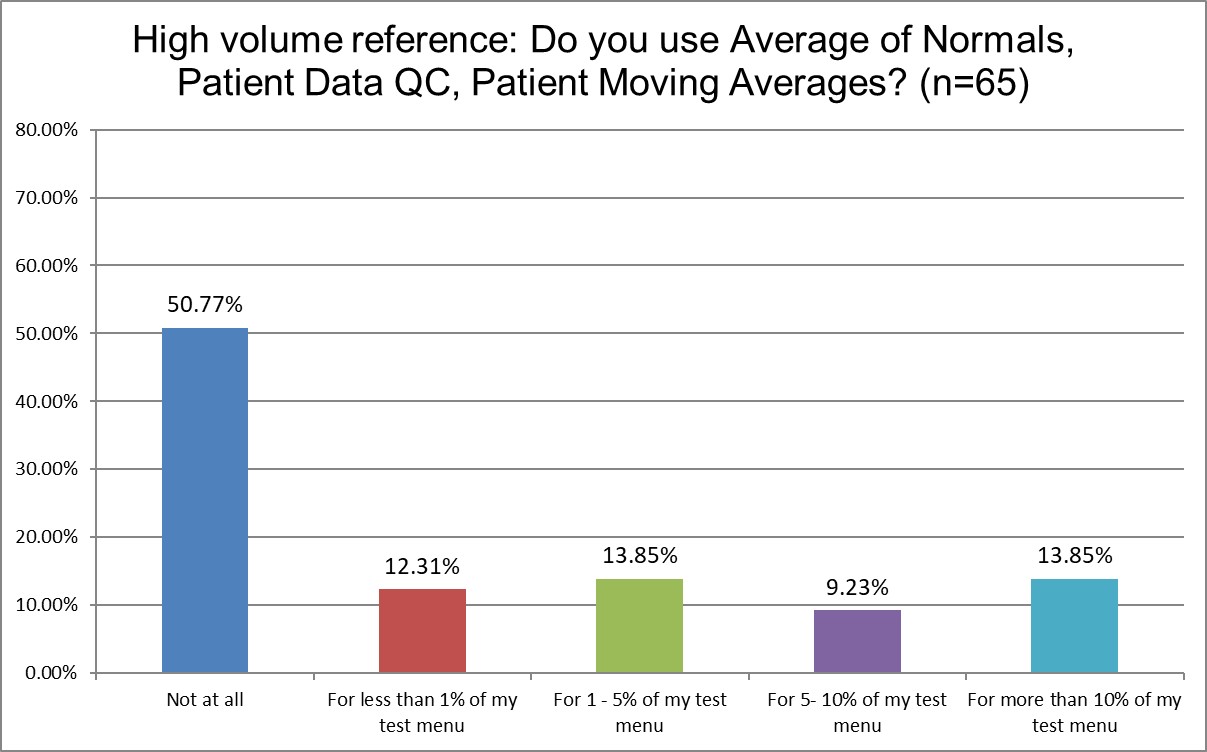
Public labs are more likely to use Patient Data QC than Private labs (about 65% Public labs don't use it at all, vs. 71.9% of Private labs). Hi Ref labs use Patient Data QC significantly more (only about 50% of Hi Ref labs don't use Patient Data QC). About 13.8% of Hi Ref labs are using it for more than 10% of their lab menu, while only 8.5% for Public, 7.2% for Private. Again, though, even for the labs that are using Patient Data QC, they use it on a very small proportion of their menu.
The Frequency of Running Controls
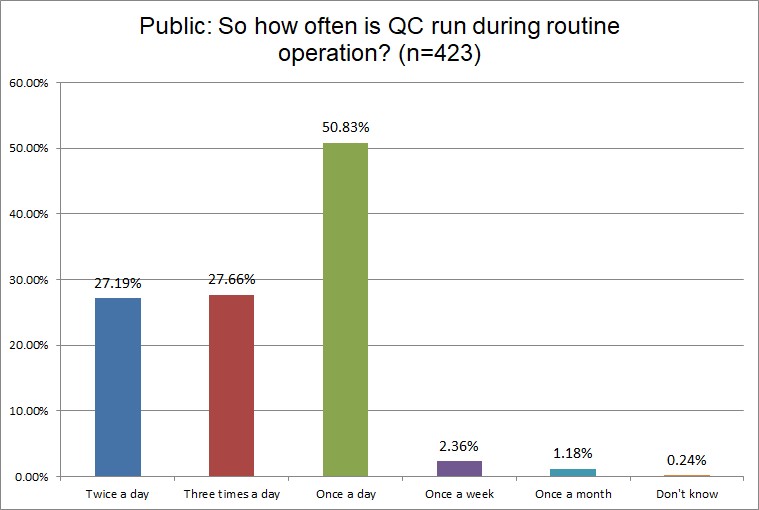
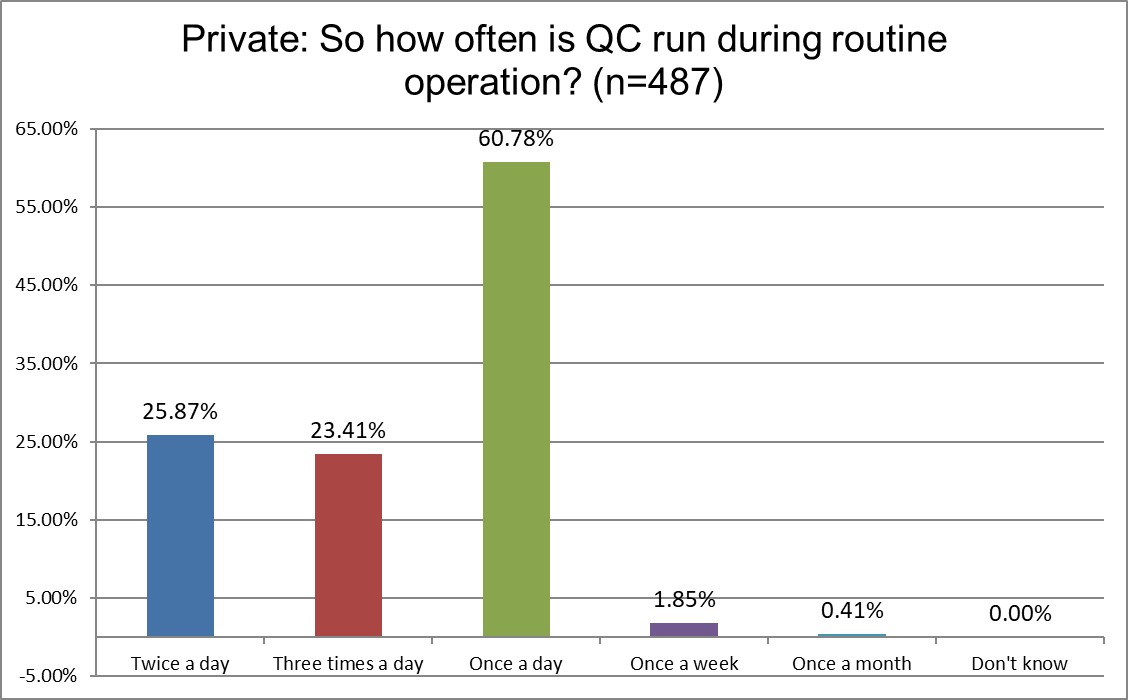
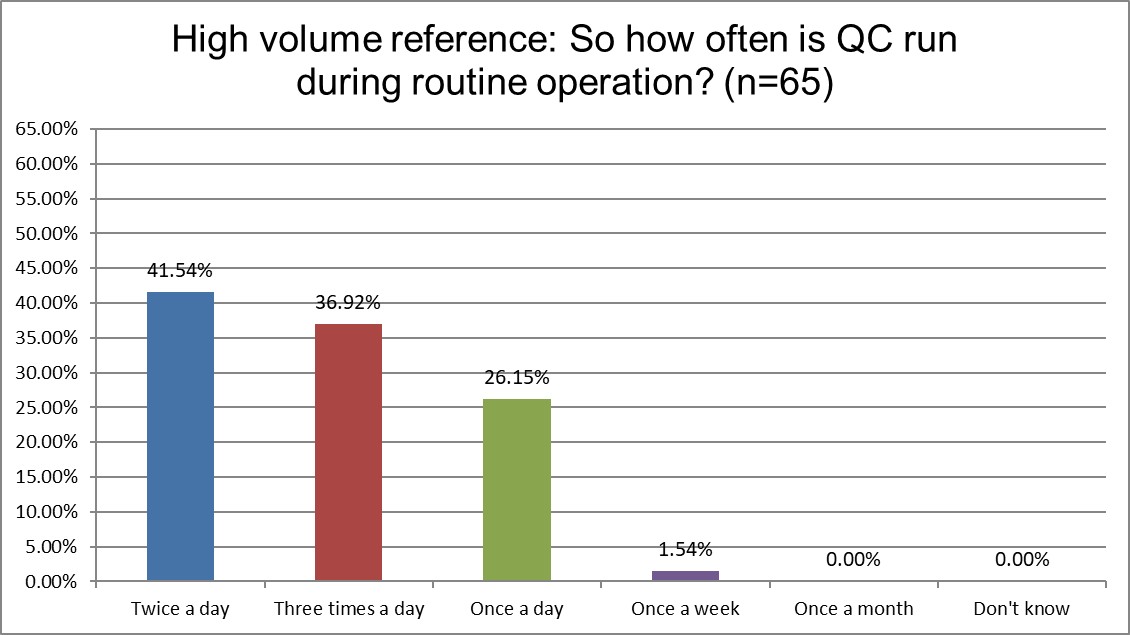
Hi Ref labs are most likely to be running QC twice a day, while a majority of both Public and Private labs are only running once a day. Over a third of Hi Ref labs are runing 3x a day, while it's closer to only a quarter of Public and Private labs. Higher volume usually demands more frequent QC.
Who has more trouble when troubleshooting?
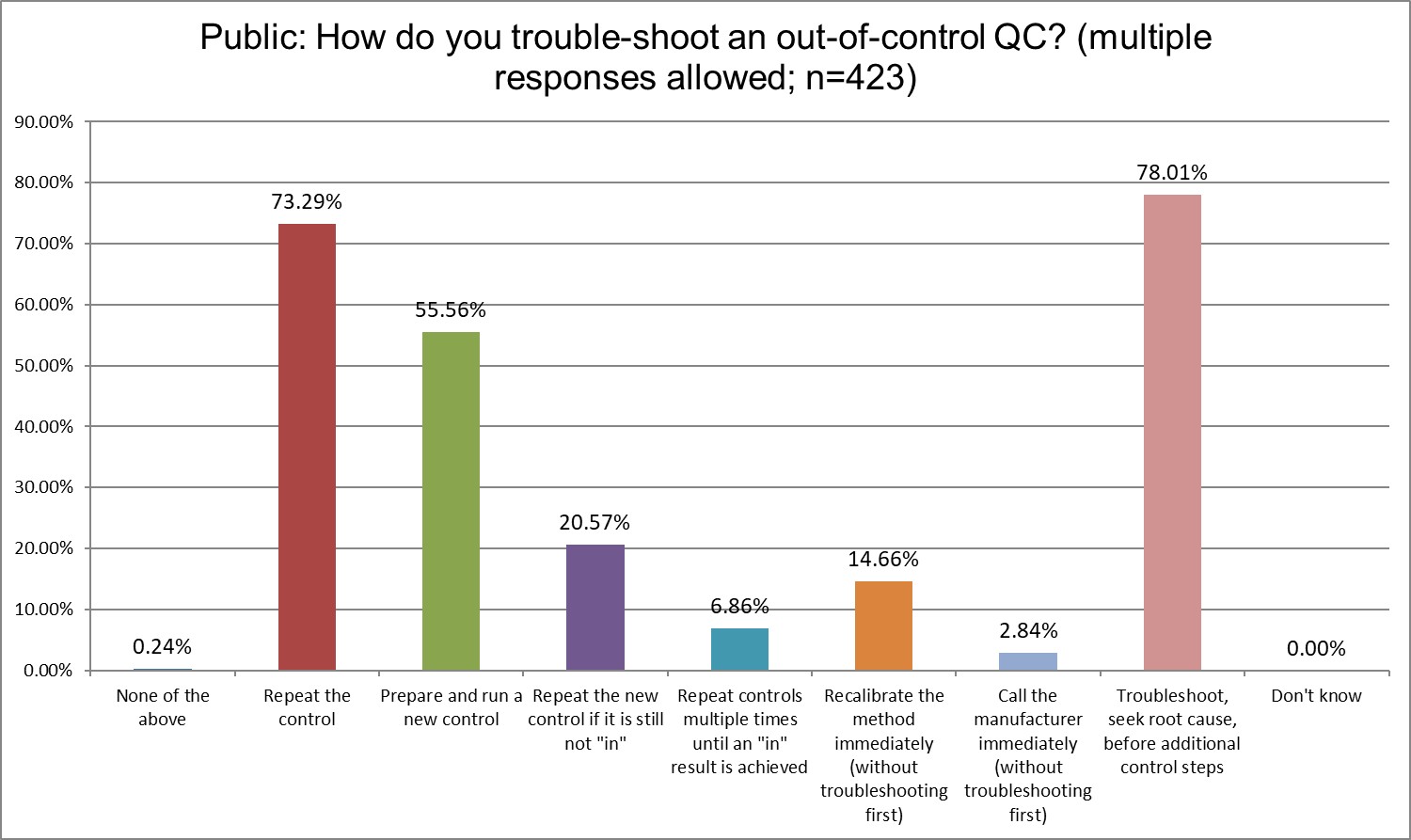
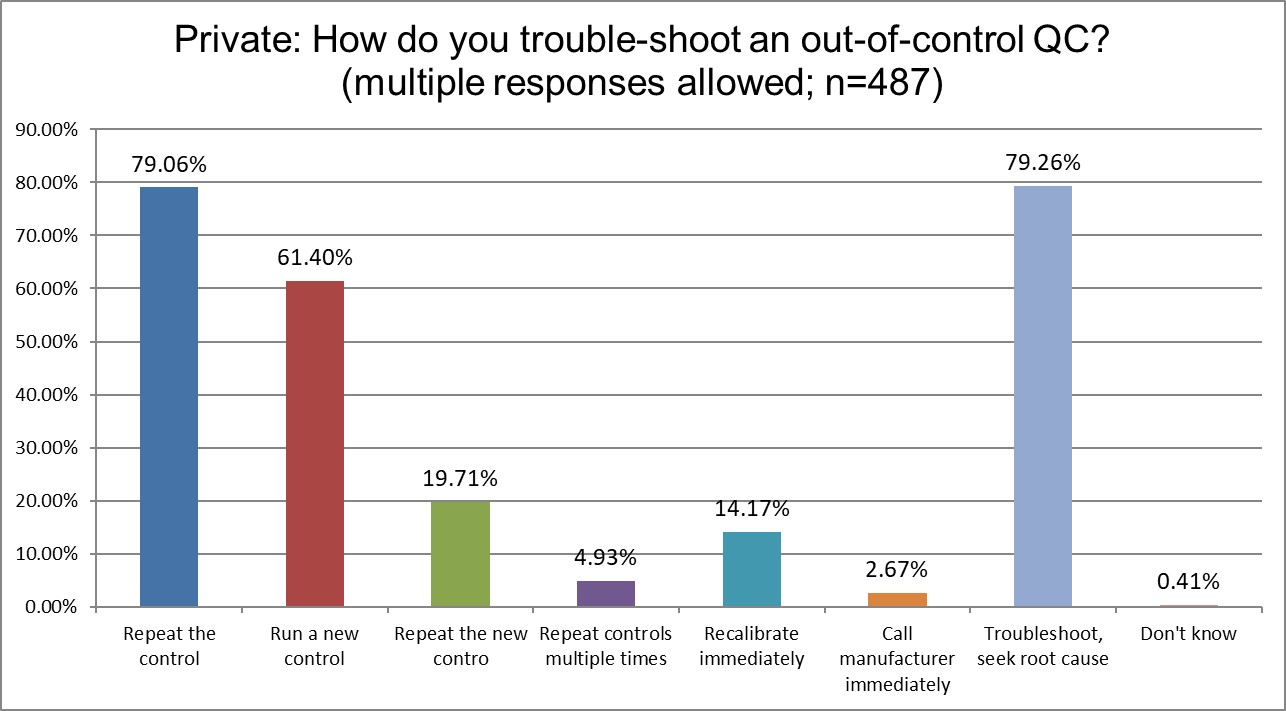
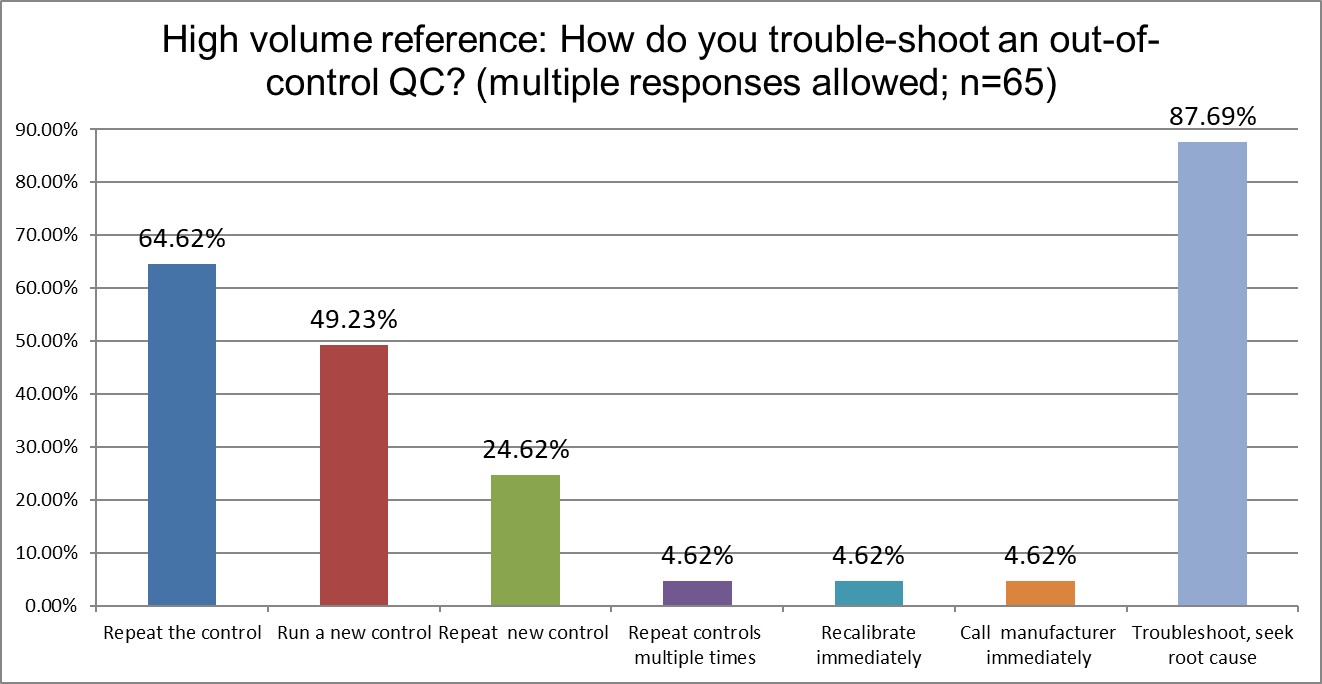
Private labs are most likely to repeat the control, High Ref labs are the least likely. Running a new control also varies significantly, with Private labs most likely to do so (61% Private vs 55.6% Public vs. 49.2% High Ref). But repeating the new control is much more similar across all lab types (20.6% Public vs 19.7% Private vs. 24.6% Hi Ref). Public and Private labs recalibrate immediately significantly more often than Hi Ref labs. Public and Private labs troubleshoot and seek root cause at about the same rate (78% Public, 79.3 % Private), while this is more prevalent in Hi Ref labs (87,7%)
Who's out of control more often? Public, Private or Hi Ref labs?
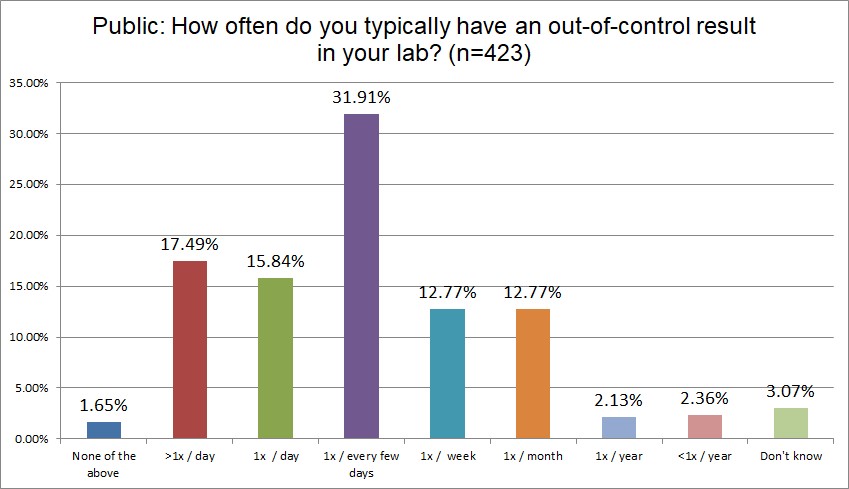
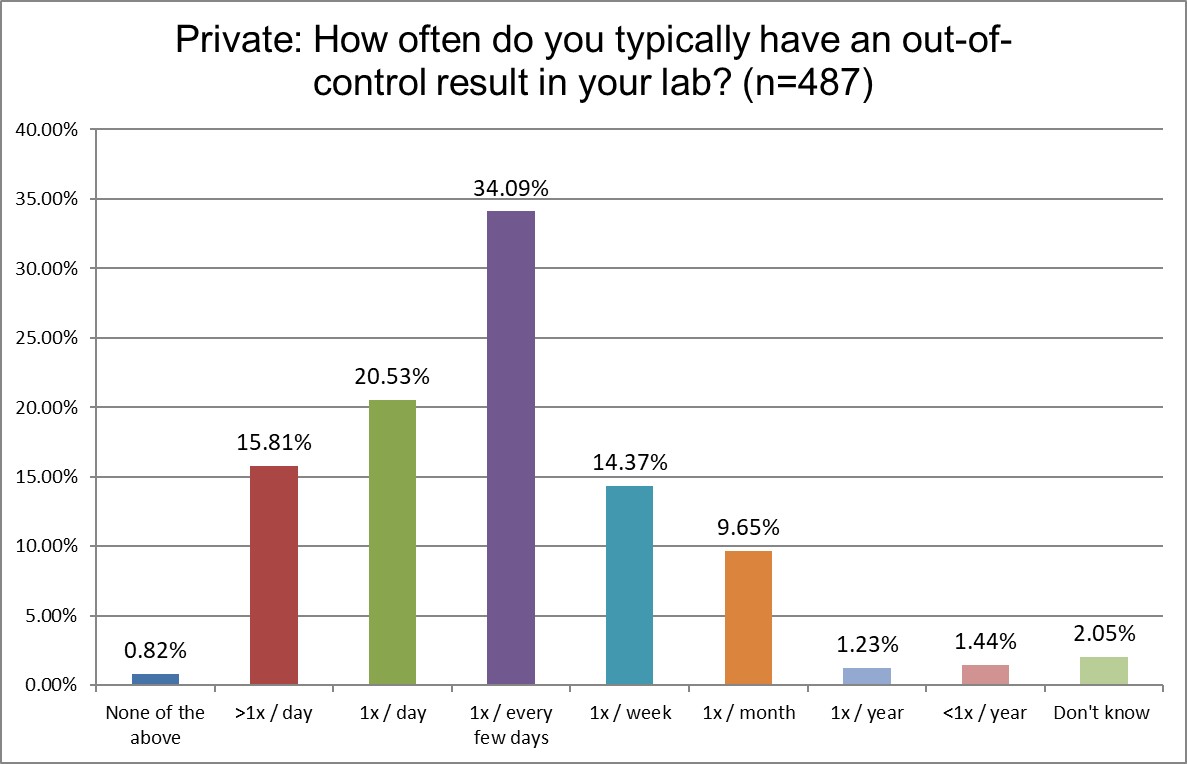
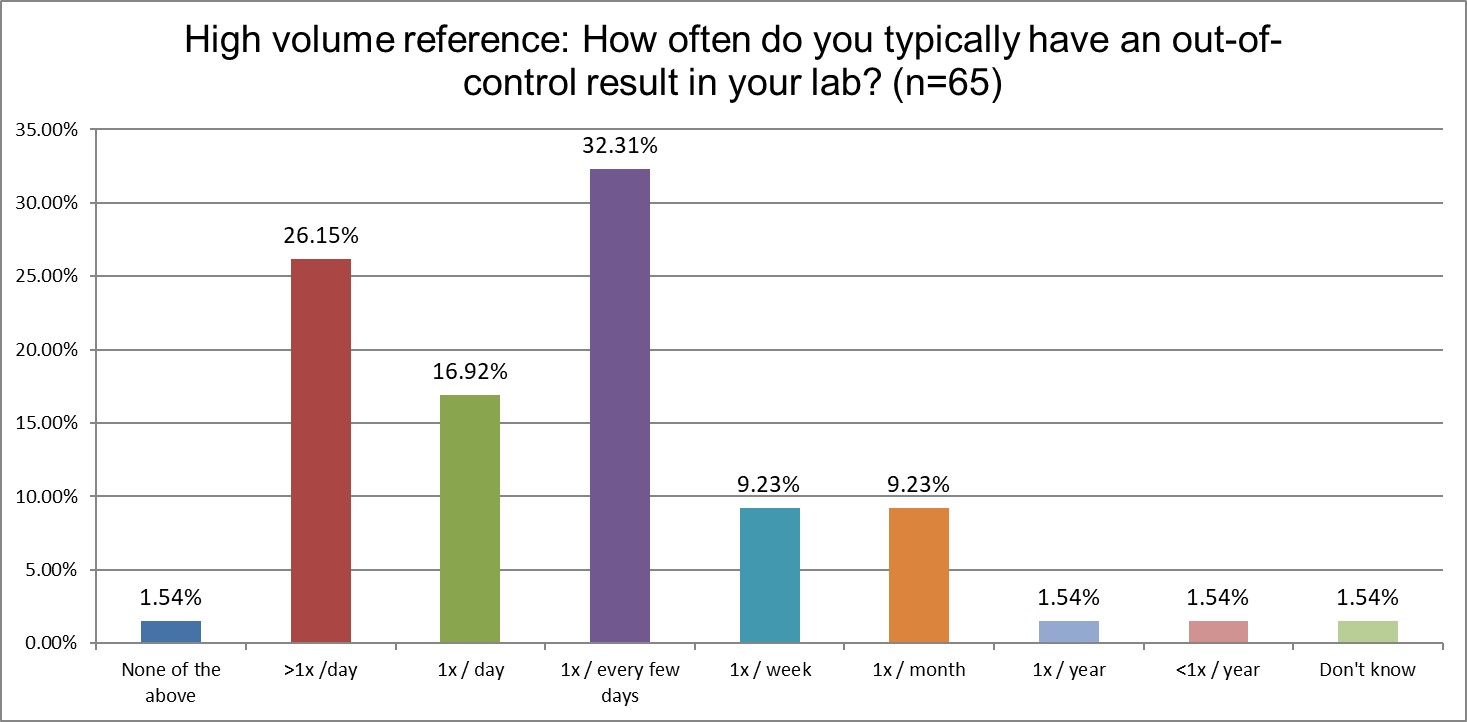
Hi Ref labs are far more likely to be out-of-control every day, or multiple times per day, than Public or Private labs (43.1% Hi Ref vs. 36.3% Private vs. 33.3% Public). Hi Ref labs lead in being out-of-control multiple times per day, as well as once per day. About a third of all types of labs are out-of-control every few days. Private labs are slightly more likely to be out-of-control only once per week. For all the differences we see in QC practices, the math is simply unforgiving: if you run more tests, and run more QC for those tests, you will have more false rejections (with a few true outliers hidden among the crowd). No matter what kind of lab you are, if you are using the wrong SD, the wrong rules, false rejection gets you in the end.
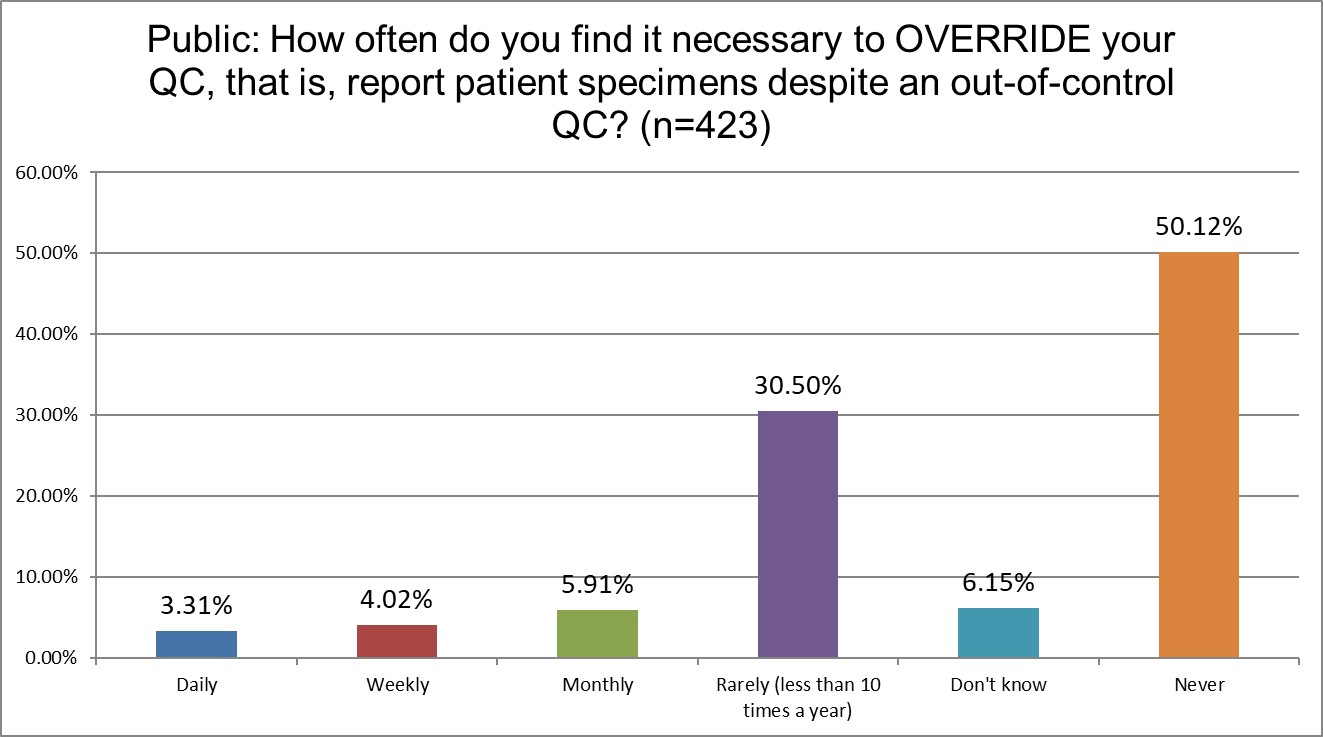
How likely are Public, Private and Hi Ref labs to override their own QC?
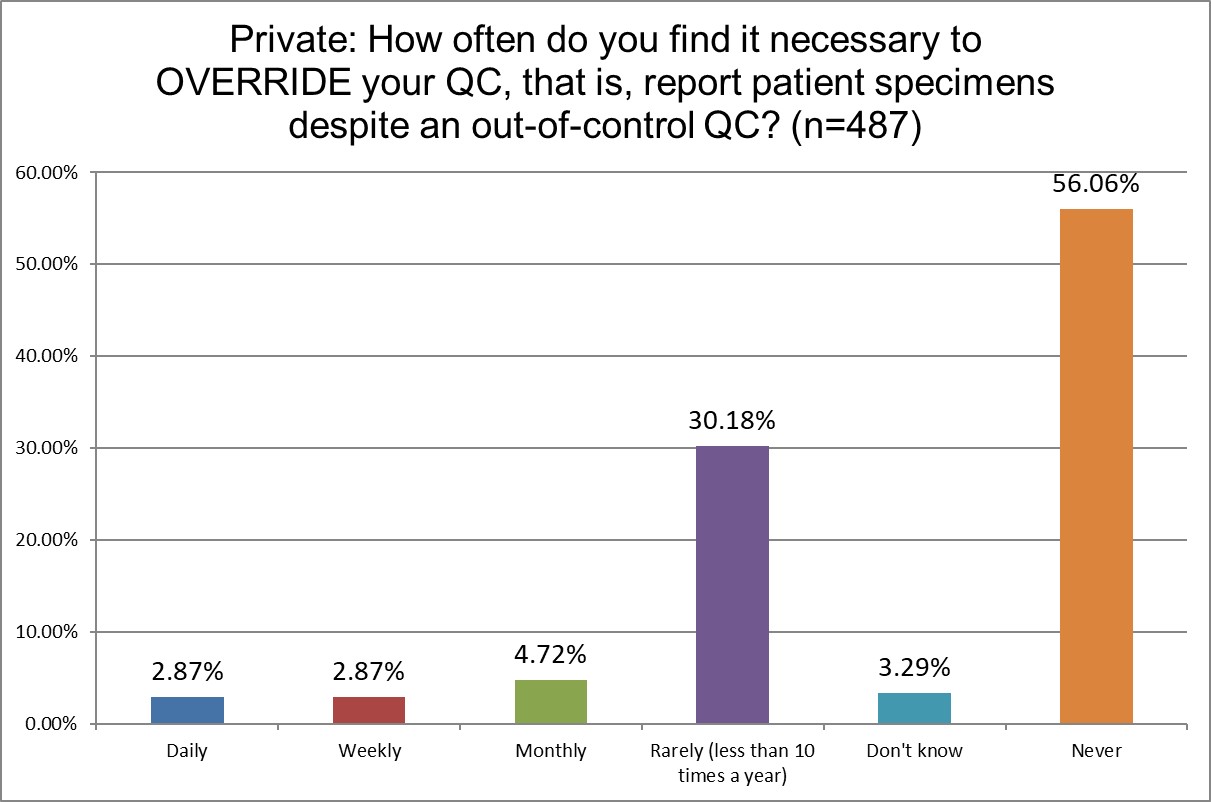
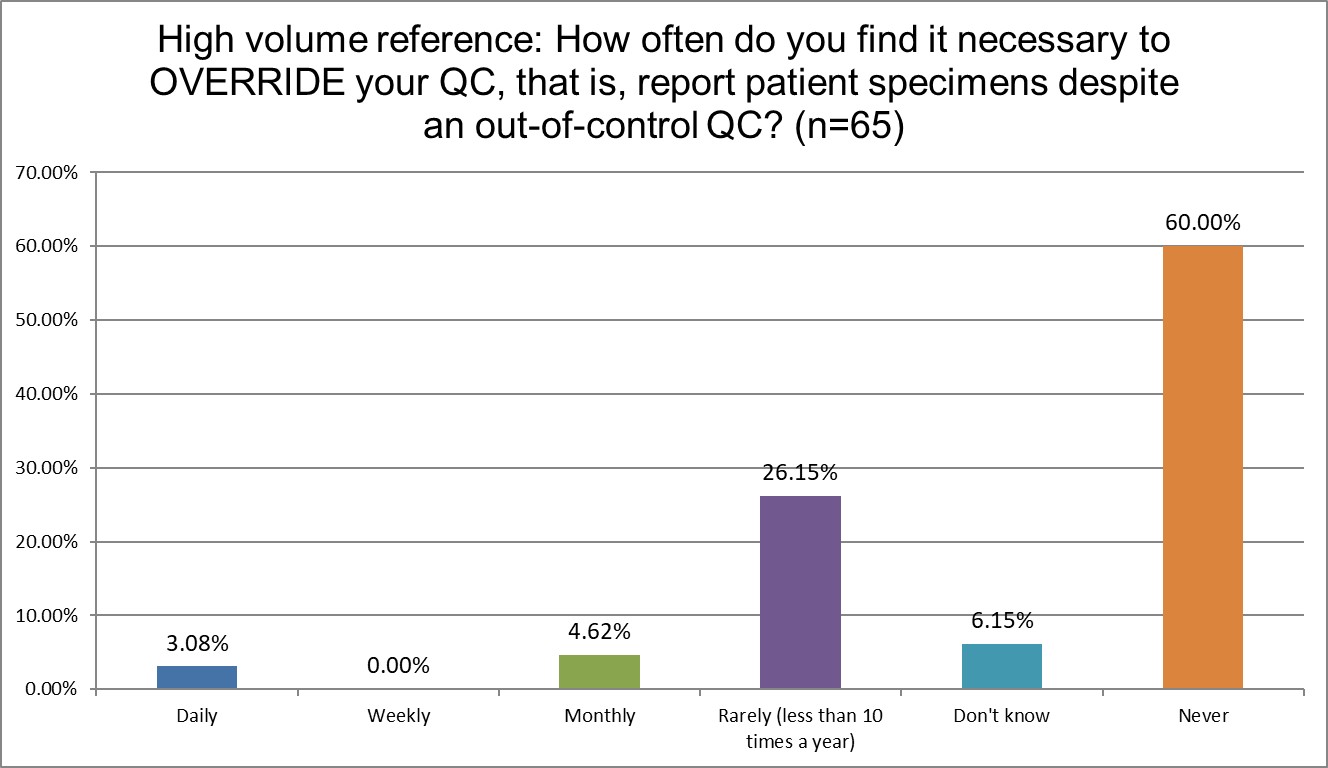
Public labs are almost twice as likely to override their own QC as Hi Ref labs (13.24% Public vs 7.7% Hi Ref). Private labs are somewhere in-between at 10.46%. Hi Ref labs are also more likely to have never overriden their QC results.
How likely are labs to correct for bias in their uncertainty?
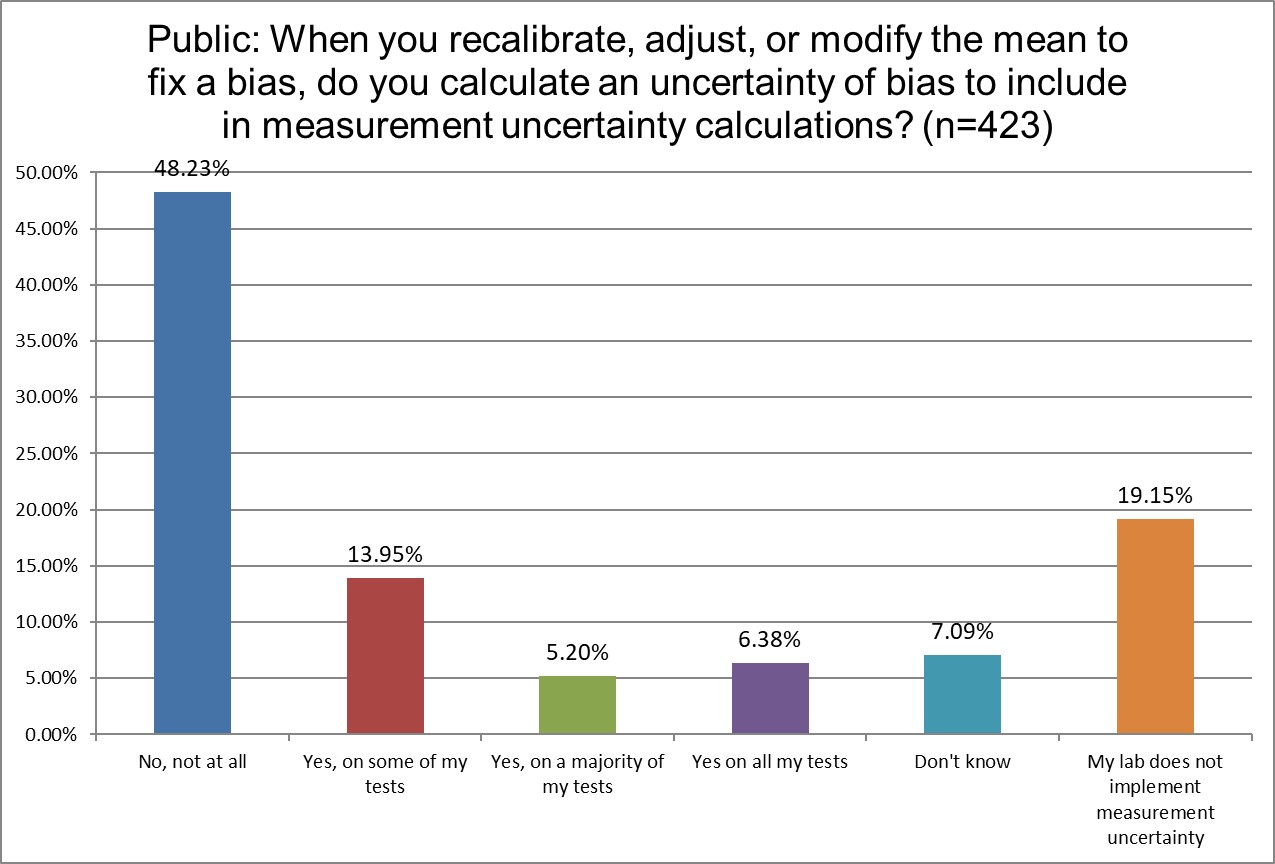
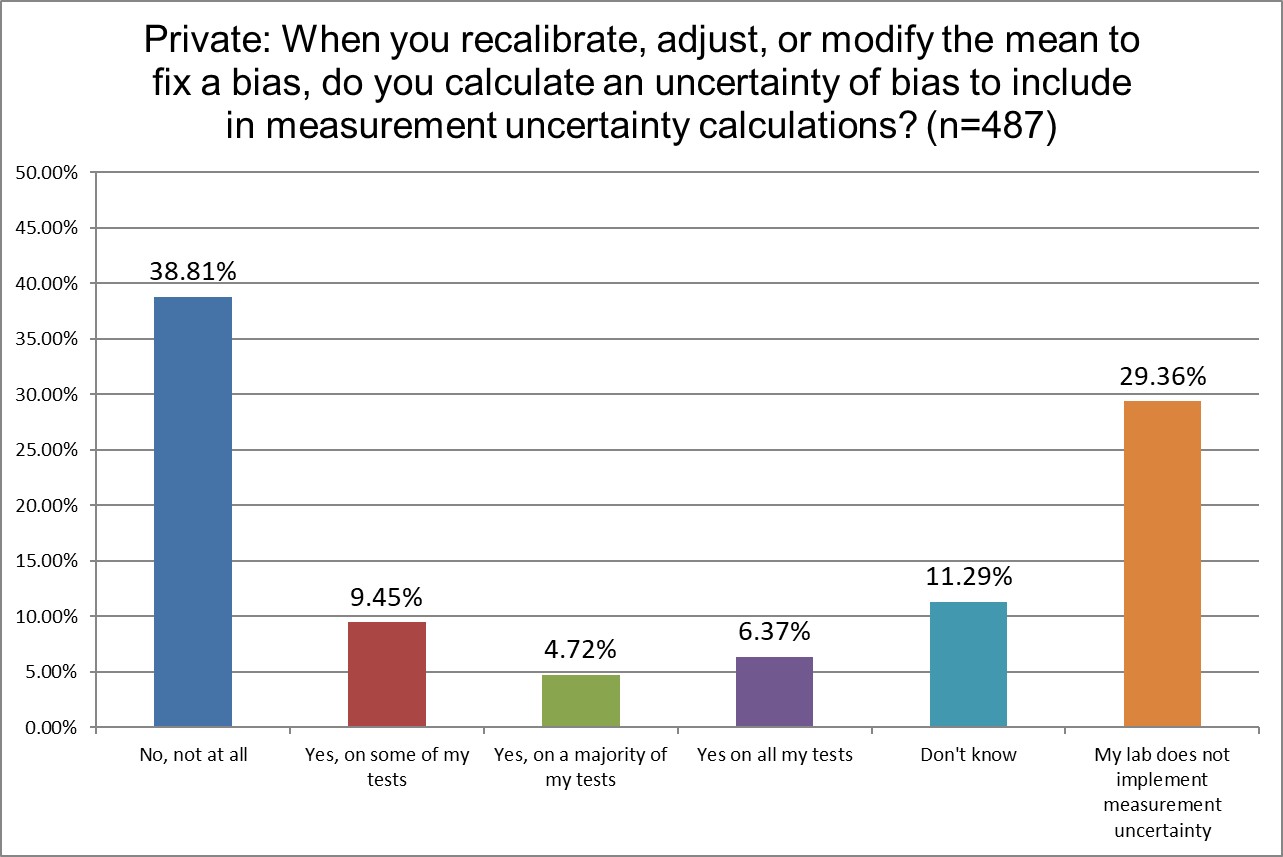
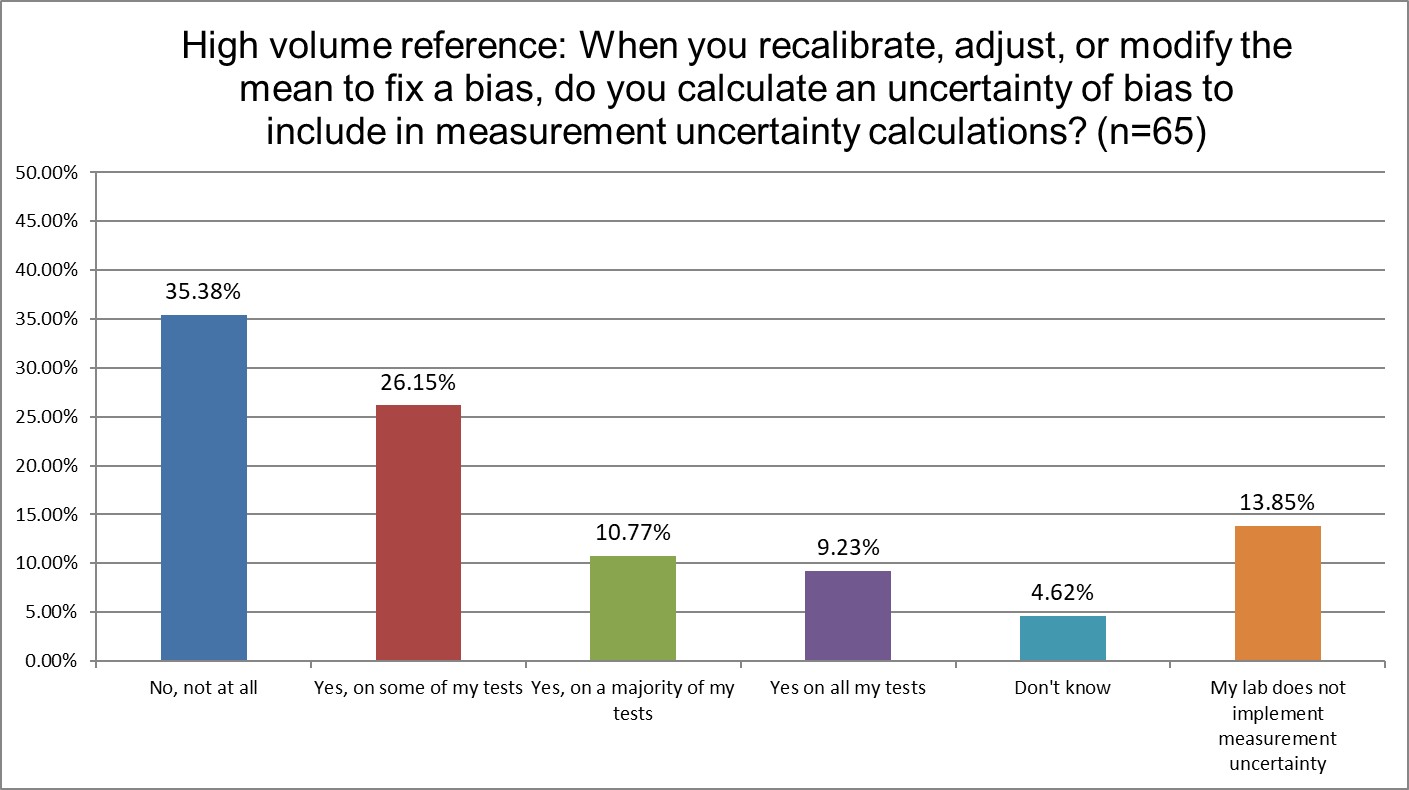
Hi Ref labs are the least likely to not be implementing measurement uncertainty.
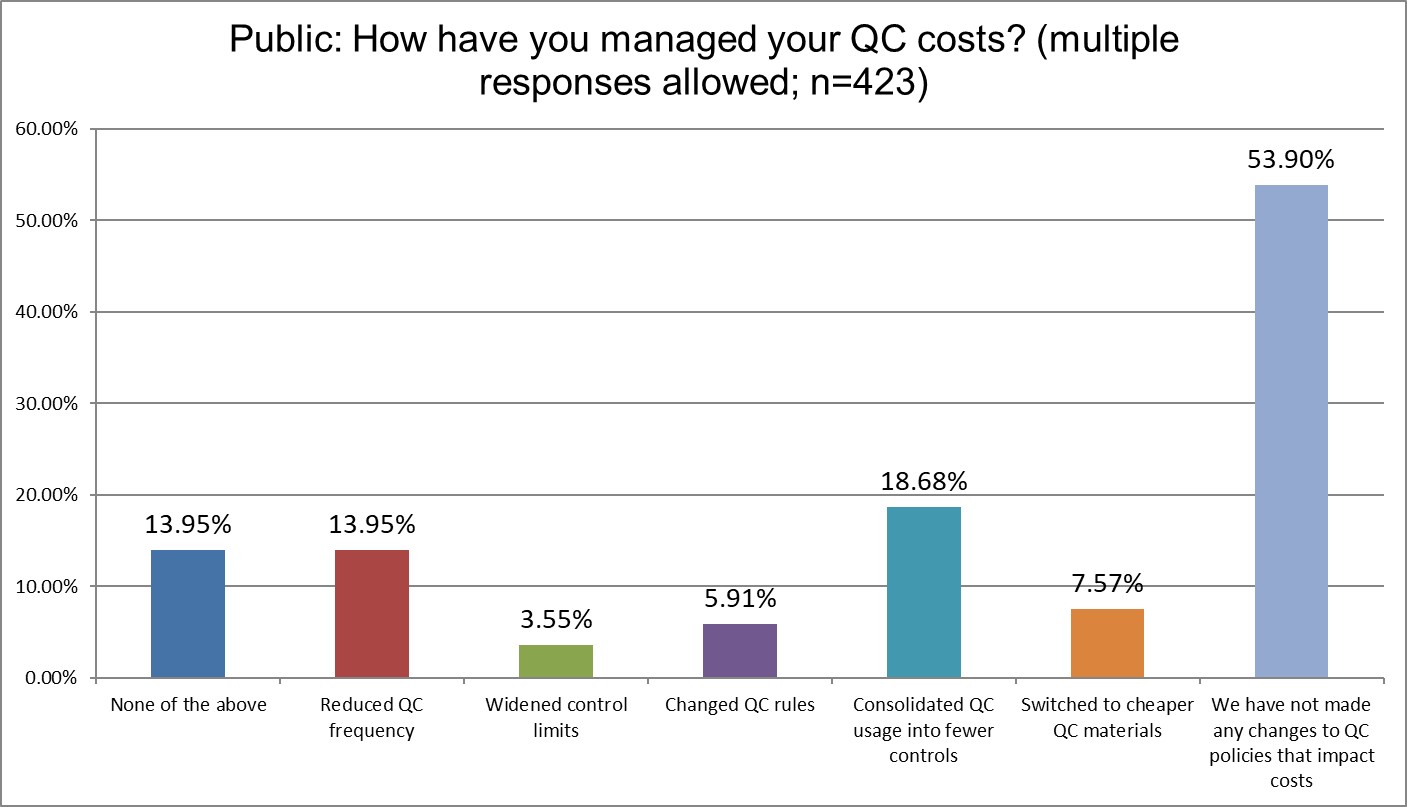
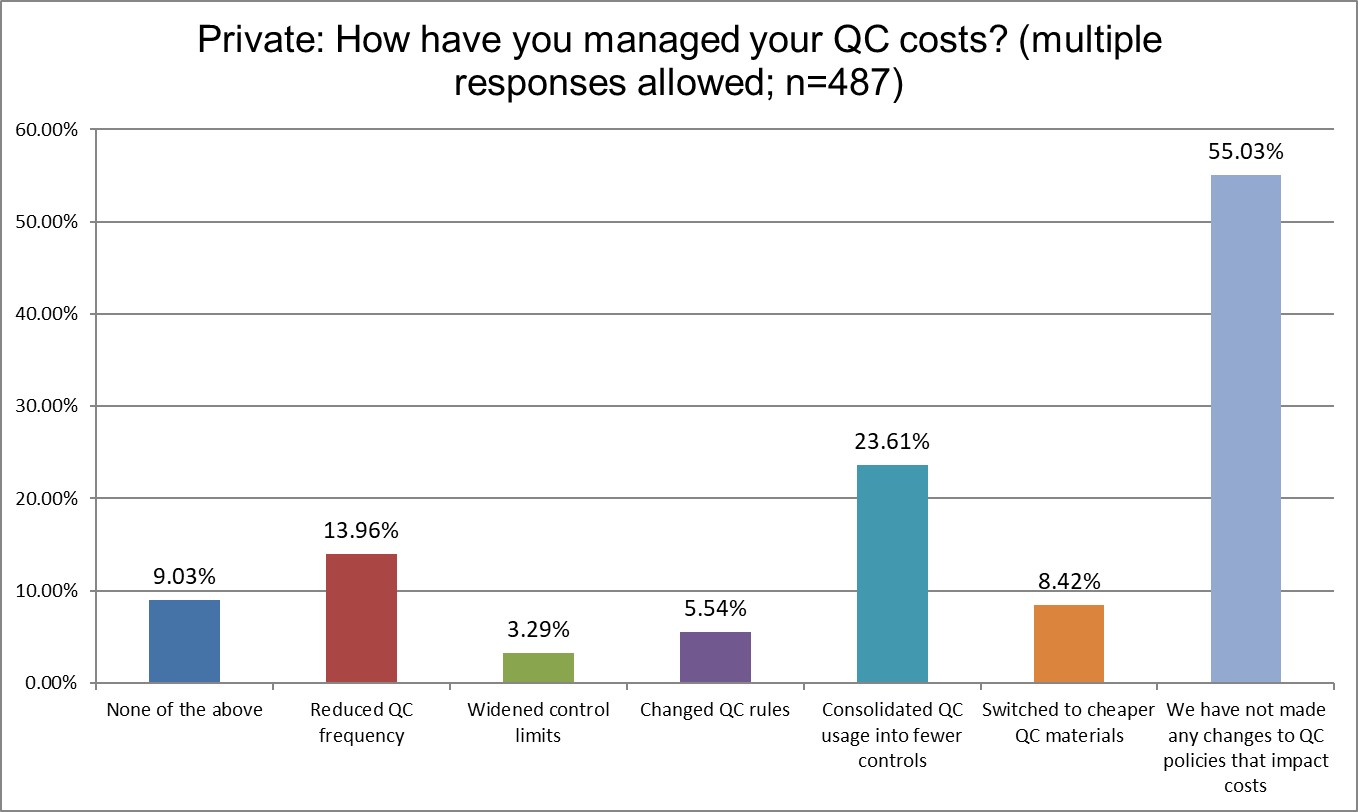
The Final Overview
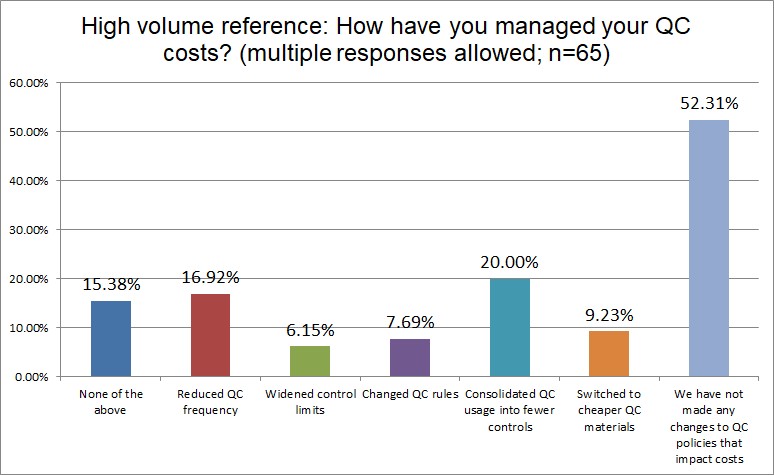
A majority of all lab types have done nothing to manage their QC costs. The dominant control vendors enjoy their padded profits because of it. Hi Ref labs are almost twice and likely as other labs to have widened their control limits (given how many outliers they have suffered, this is not much of a surprise). Widening control limits is what we derisively refer to as Blind Man QC. It's creating an illusory Levey-Jennings chart that makes QC look in control, even when it's not. Private labs are more likely to have consolidated their QC, Public labs are least likely to have done so. Changing QC rules, one of the easiest options for a laboratory, has been done by less than 10% of all lab types.
Conclusion
There is no type of laboratory that is immune from bad practices. But there do seem to be some interesting habits that predominate in some types.
Public labs have the worst rate of overriding their own QC, are most likely to use manufacturer QC and manufacturer ranges.
Private labs are most likely to use 2 SD limits, most likely to use 2 SD for rejection, and are (not surprisingly) the most likely to repeat controls.
Hi Ref labs have the worst frequency of being out of control, and are also running QC the most often. They lead in the use of PBRTQC and the highest rates of using use Westgard Rules.
All types of laboratories are plagued with bad habits. They can all learn to do better.