Basic QC Practices
The Global QC Survey Results: High vs Low Volume Laboratories
The Global QC Survey of 2021 collected responses from both high volume and low volume laboratories. Are there any differences in how they implement QC? Are there any differences in how they experience QC outliers?
The Global QC Survey Results: Do High Volume and Low Volume Laboratories operate and experience QC differently?
Sten Westgard, MS
March 2022
[This survey was completed with the support and partnership of Technopath Clinical Diagnostics.]
- The Top Ten Findings of the 2021 Global QC Survey
- The 2021 Global QC Survey
- The 2021 US QC Survey Results
- The 2021 Asia QC Survey Results
- The 2021 Middle-East QC Survey Results
- The 2021 Latin and South America QC Survey Results
- The 2021 European QC Survey Results
The difference between high volume and low volume
For this analysis, we looked at laboratories that had volumes less than 100,000 a year (hereafter referred to as "low volume laboratories") and laboratories that had more than 1,000,000 a year (hereafter referred to as "high volume laboratories") . One could reasonably argue that 100,000 tests a year is still pretty high in volume, but for this survey we're focusing on the highest volume labs vs the not-so-highest volume labs.
The predominant focus of these labs' answers was on chemistry. Both groups also had a similar dominant regulatory structure, ISO 15189. There are some common assumptions we make about high volume laboratories that may be challenged by the survey results.
[note: in the following graphs, the first one presented is for high volume laboratories, followed by low volume laboratories. The text of the questions and answers are condensed for better readability in this article, but the questions and answer choices were exactly the same when the laboratories filled out the survey.]
The QC Set Up
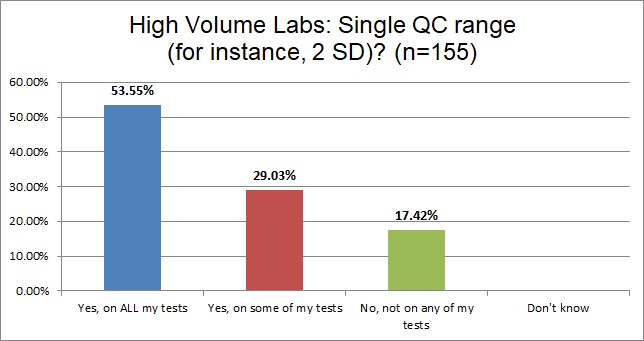
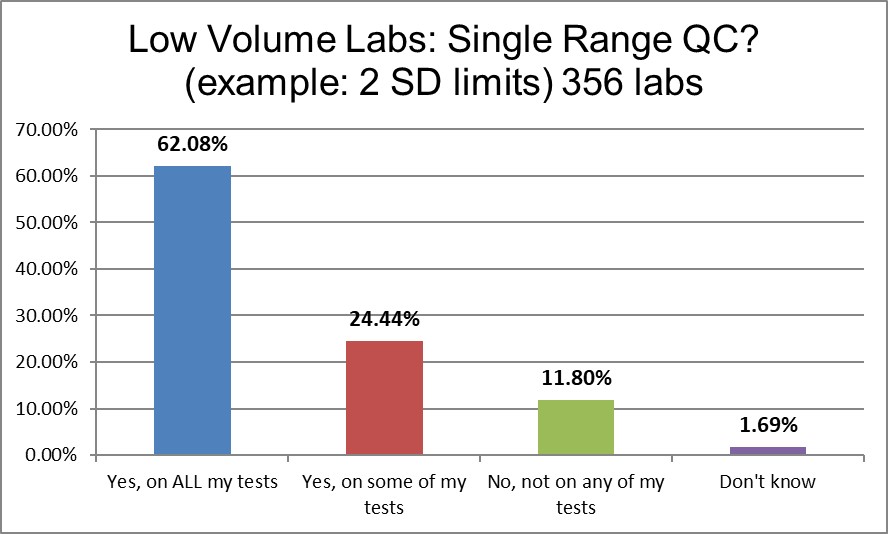
Lower volume laboratories are significantly more likely to use 2 SDs on all their tests.
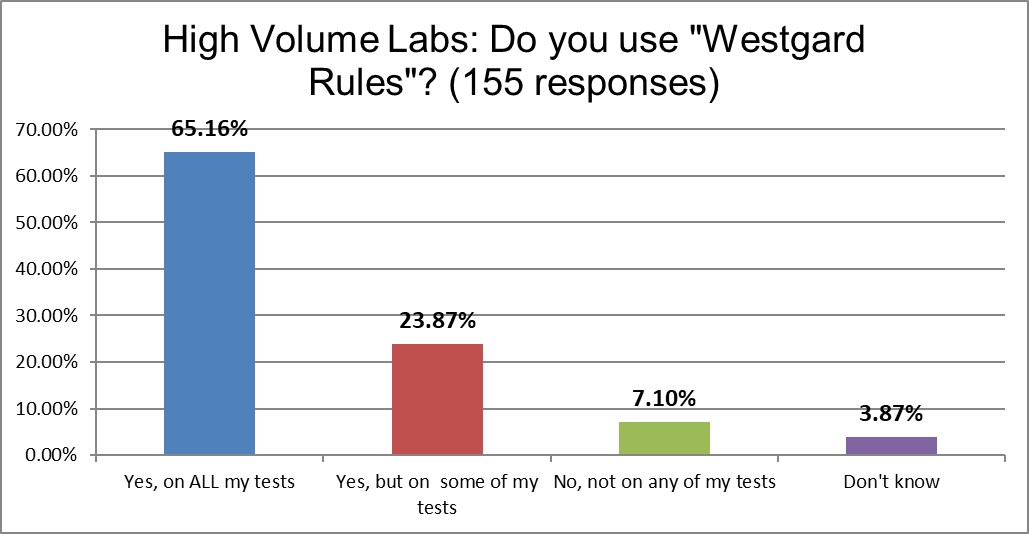
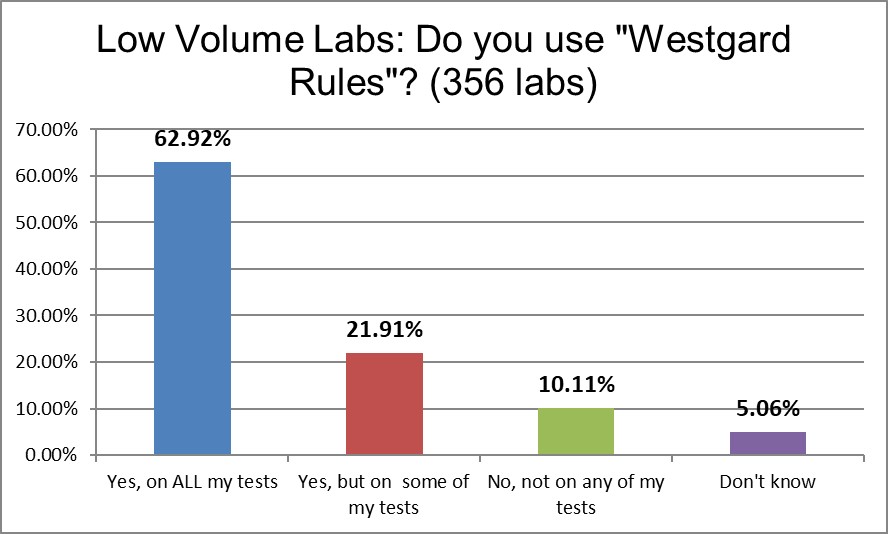
Both groups have a high use of "Westgard Rules" on some or all of their tests. High Volume laboratories have a slightly higher use rate (89%) vs Low Volume laboratories (85%). That's not a large discrepancy. It seems Westgard Rules are not only universal across countries and regions of the world, but also across different volumes of testing.
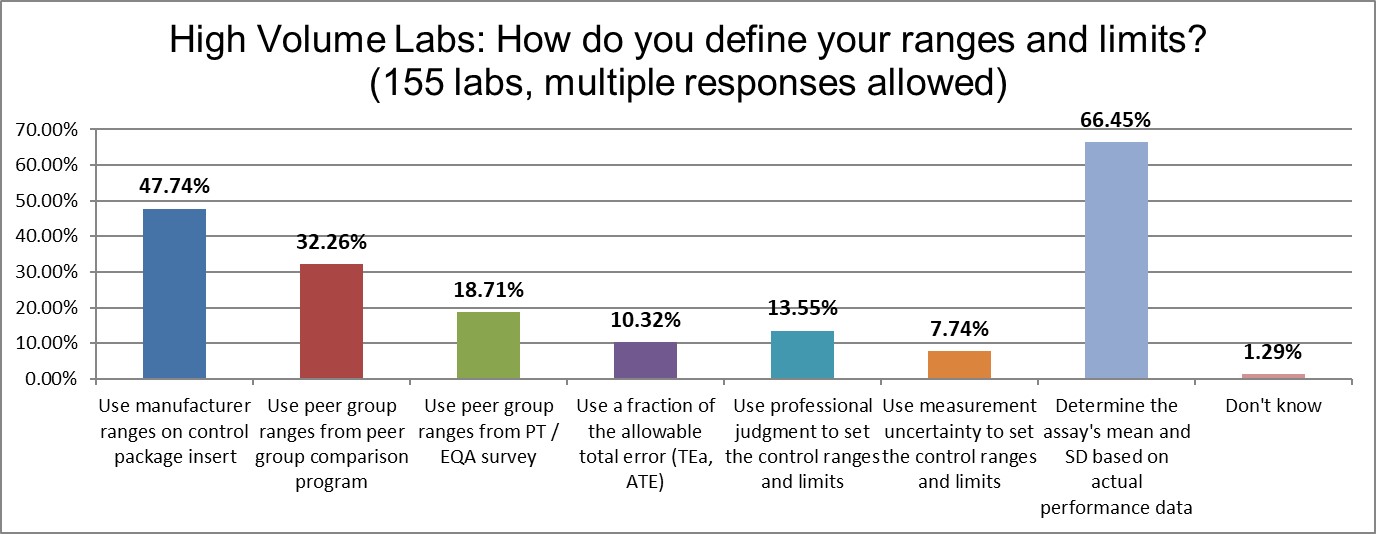
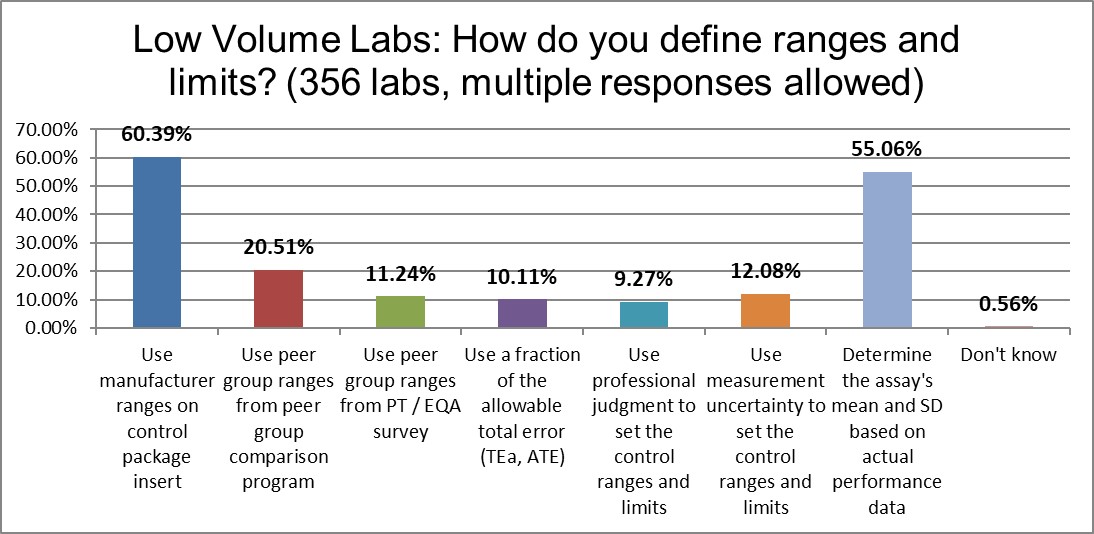
When it comes to setting control range and limits, the significant difference between high and low volume laboratories reveals itself most in the use of laboratory's own calculated mean, where high volume laboratories do it more (approx. 66% vs 55%). In contrast, low volume laboratories use more manufacturer-defined ranges (60% vs 47%). The observation to be made here is perhaps the high volume laboratories have more resources and focus to focus on their own performance, not the package inserts.
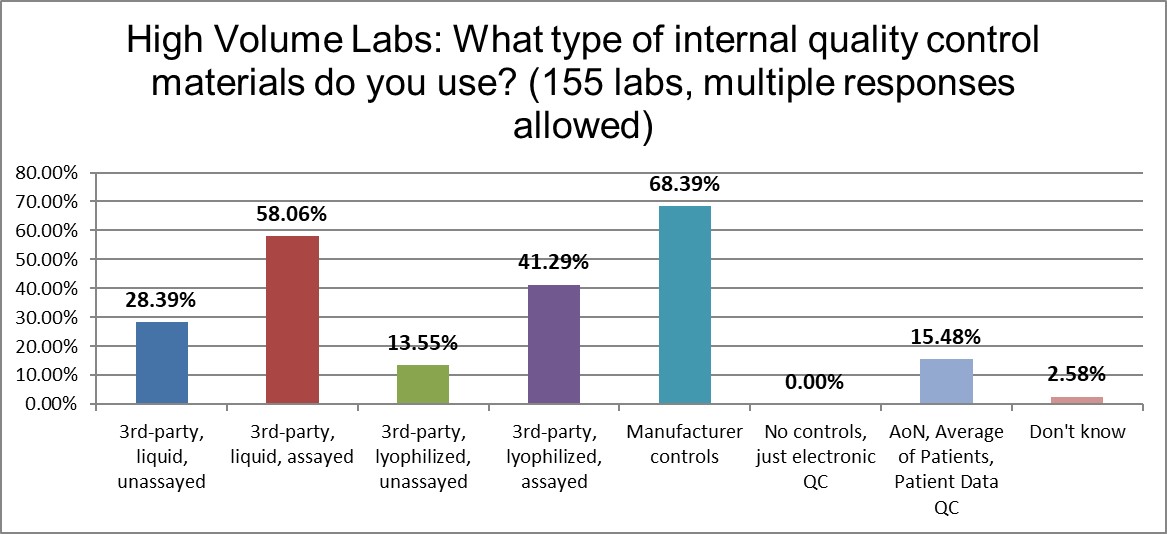
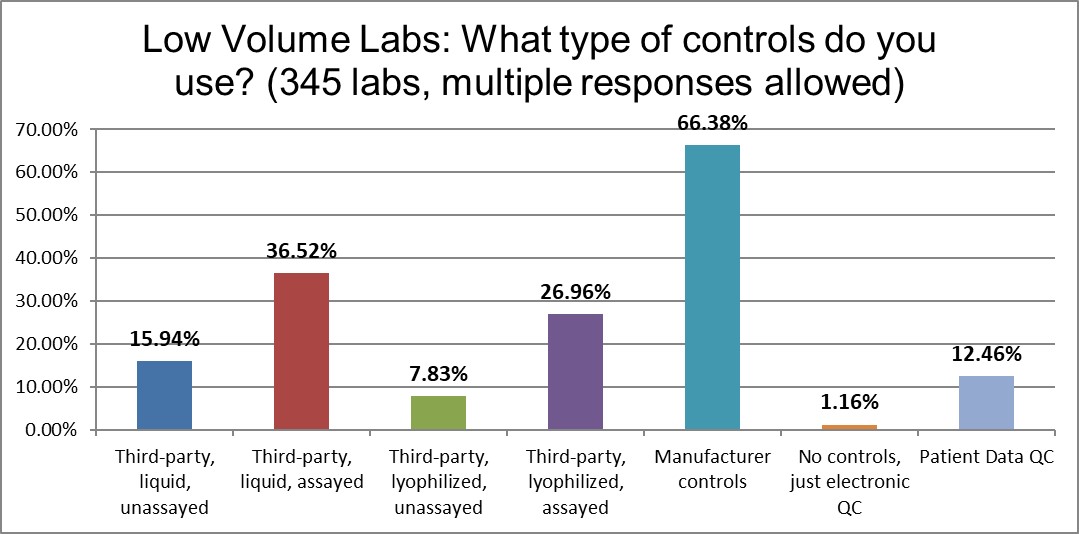
When it comes to the use of manufacturer controls, there really isn't much difference between high and low volume laboratories (68% vs 66%). There is more of a difference in the use of 3rd party lyophilized assayed controls (41% to 26%, high v low) and 3rd party liquid assayed controls (58% to 36%, high v low). There is more use of liquid controls occurring in the high volume laboratories, perhaps indicative of better resources.
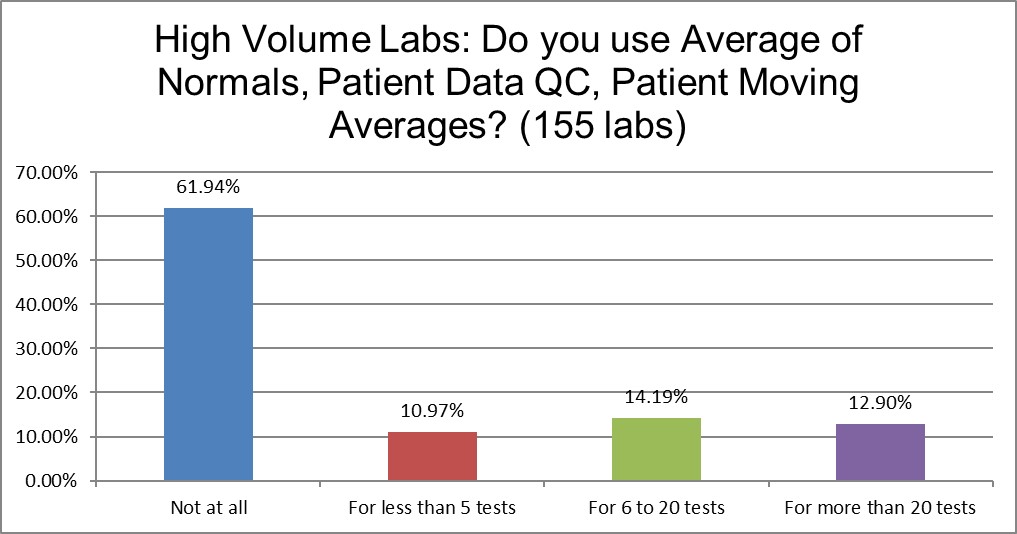
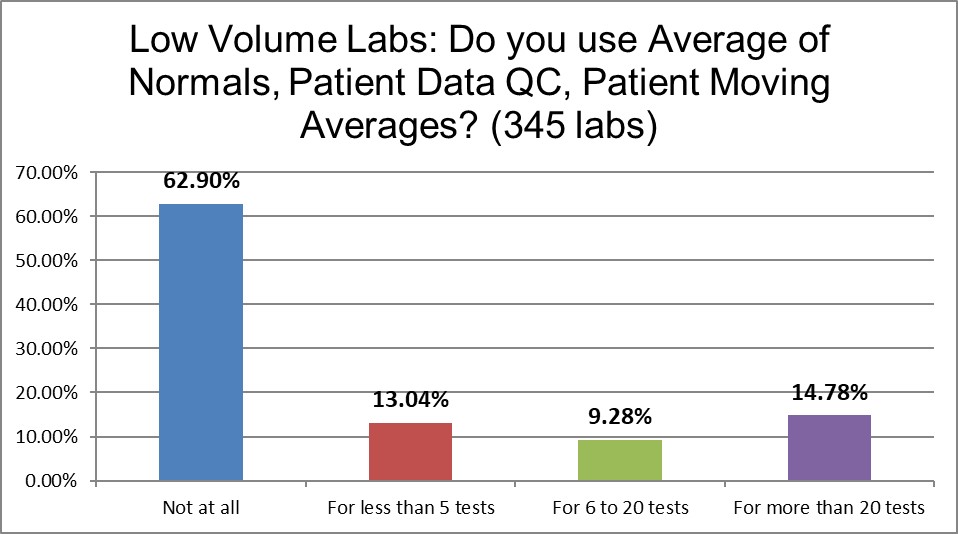
Nearly two-thirds of laboratories of both kinds of labs use no patient data techniques at all. Where one might assume the higher volume laboratories have more resources and skills to implement the Patient Data techniques, there is almost not difference between the high and the low. Perhaps conventional wisdom has assumed that high volume laboratories are using more patient data techniques, but that's not bourne out by the facts. Labs at any volume are just as likely to avoid spending time on these techniques.
The Real Practice of Running Controls
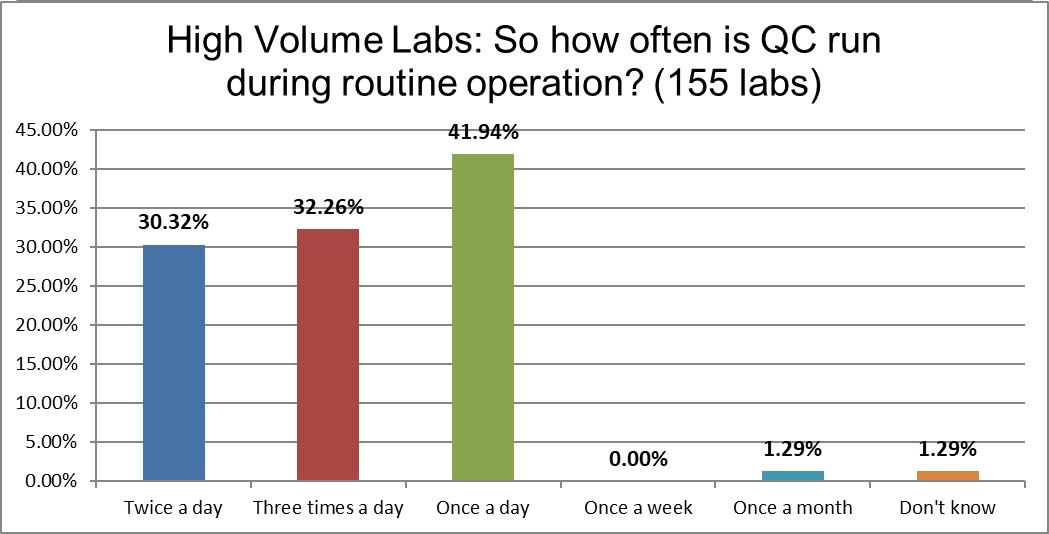
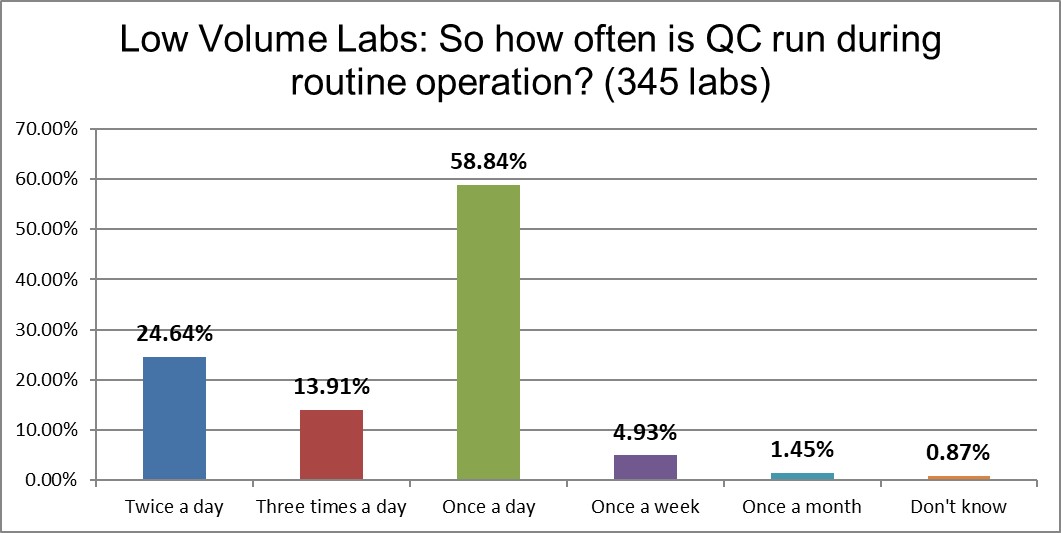
Once-a-day QC is the long favorite of low volume laboratories, whereas high volume laboratories spend significantly more on running their QC 2 or particularly 3 times day. High volume labs are three times more likely to be running QC 3 times a day. Given their volume, that might mean the expenditure on QC is not that large on a QC per test ratio, but certainly the financial expense of QC is significantly higher in high volume laboratories. The IQCP options of running only a week or once a month are slightly more common in the low volume laboratories. (Basically, IQCP is just not that popular.)
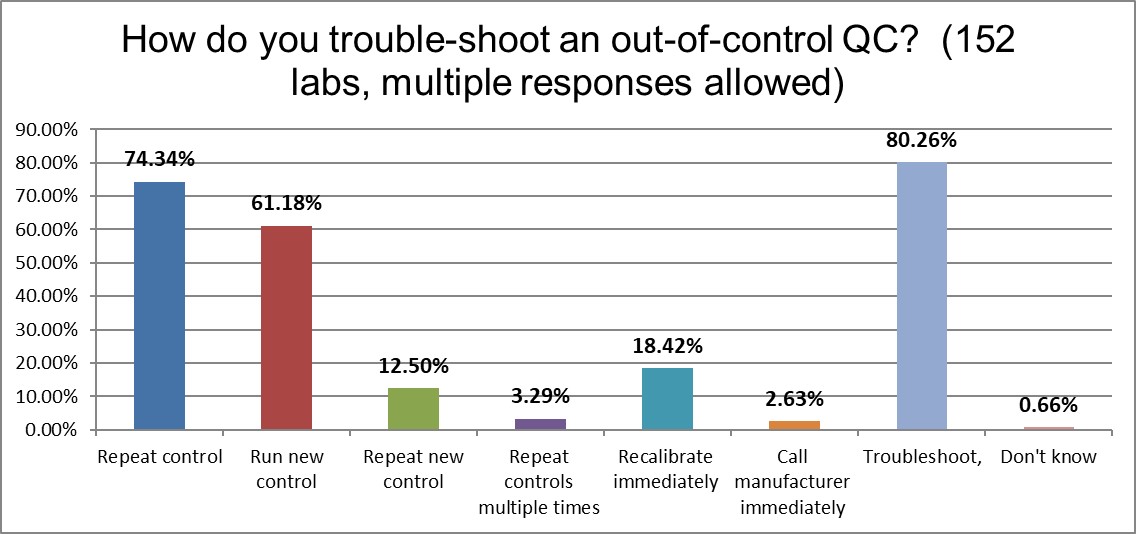
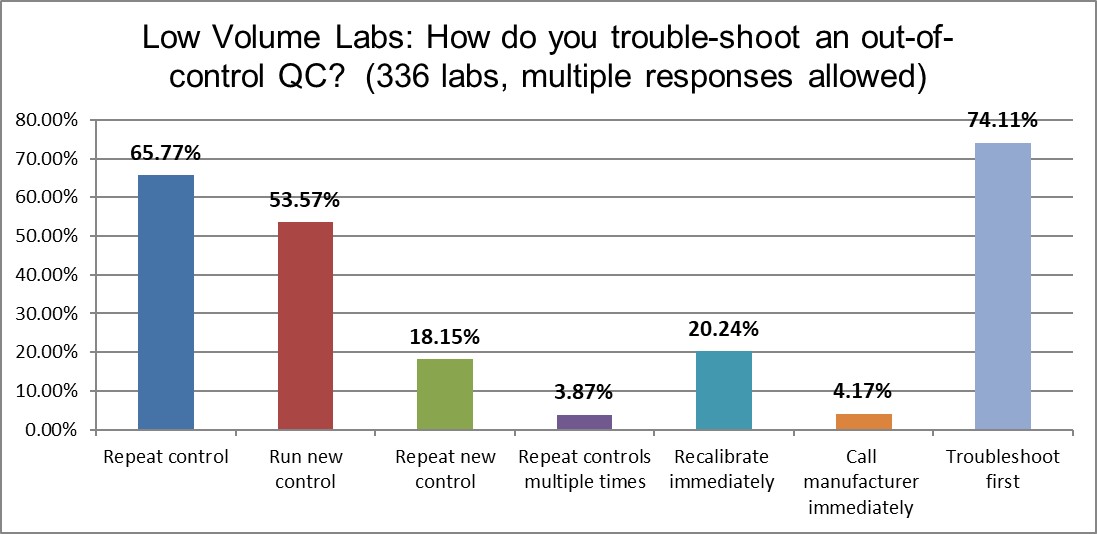
High volume laboratories are more likely to do the following this: troubleshoot first before repeating controls (80% vs 74%), repeat the control (74% vs 66%), and running a new control (61% vs 54%). The higher volume laboratories use more controls, again. It's encouraging to see that both types of laboratories try to do the right thing first (troubleshoot before repeating), but distressing to see so much repeating and repeating present in the practice. That's a lot of wasted time, money, and effort.
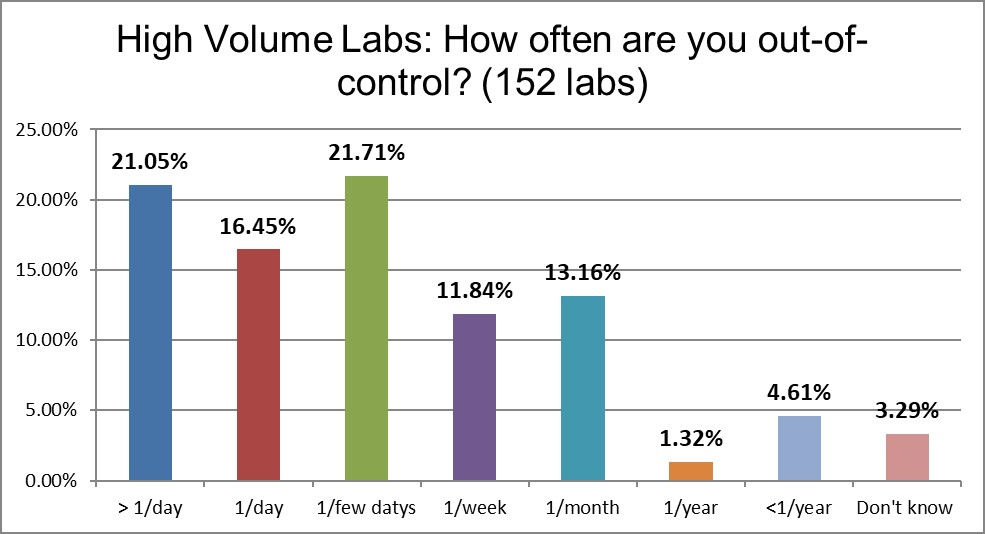
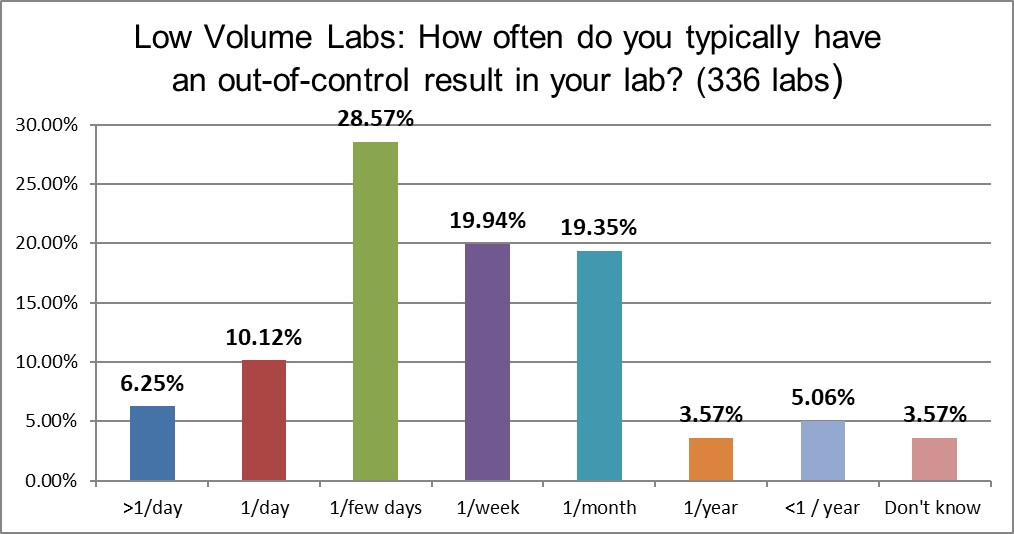
A very large difference can be seen in the frequency of out-of-control events: High volume laboratories are three times more likely to be out of control multiple times in a day (21% vs 6%) and also more likely to be out of control every day (16% vs 10%) than low volume laboratories. More tests, more problems? This leads one to begin to worry that higher volume laboratories might lose something in the scaling up.
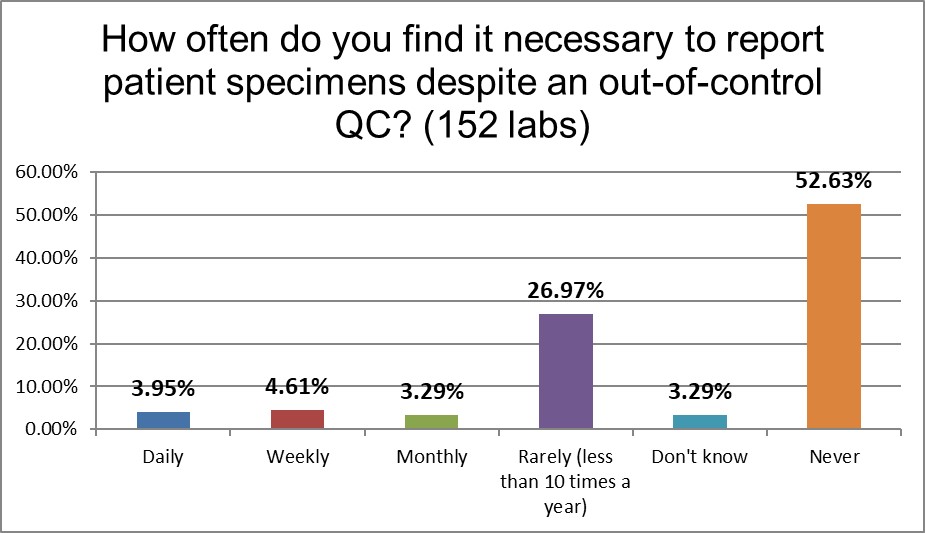
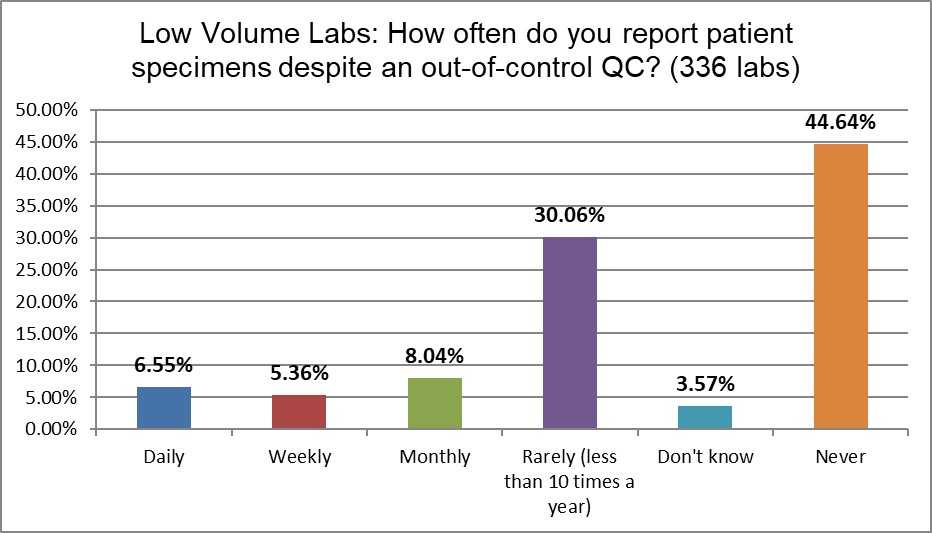
When it comes to the most worrisome habit of laboratories: releasing patient results even after the QC has indicated the run is out-of-control, an important difference is revealed: low volume laboratories are almost twice as likely to send out results (daily, weekly, and monthly combined) after bad QC (approx. 21% vs 12%). Neither rate is good, but if you're a customer of a laboratory, choosing the lab that has only a 1 in 10 chance of giving you results that are questionable, versus a laboratory that is doing this 1 out of 5 times is a pretty easy decision to make. A bare majority of high volume laboratories state they never do this, while only about 45% of low volume laboratories say the same. If high volume laboratories are experiencing more outliers, at least they are containing them better than low volume laboratories.
The Final Overview
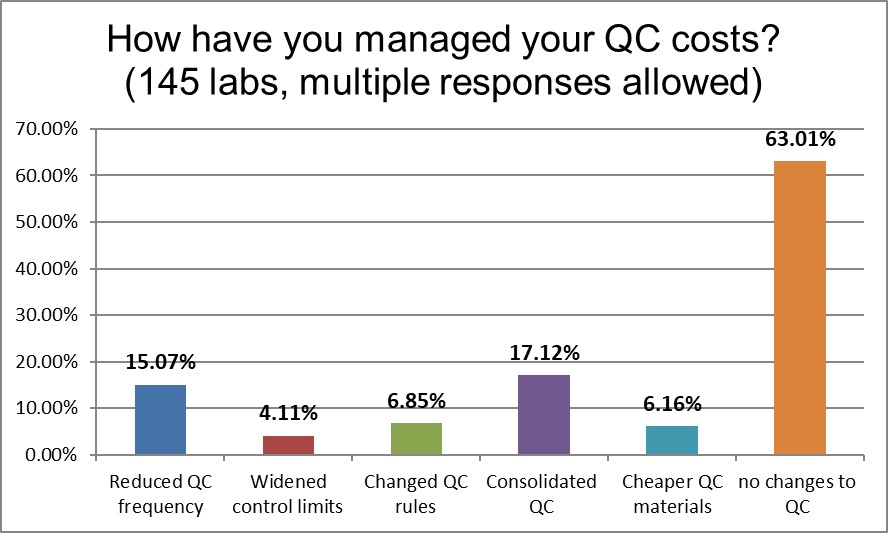
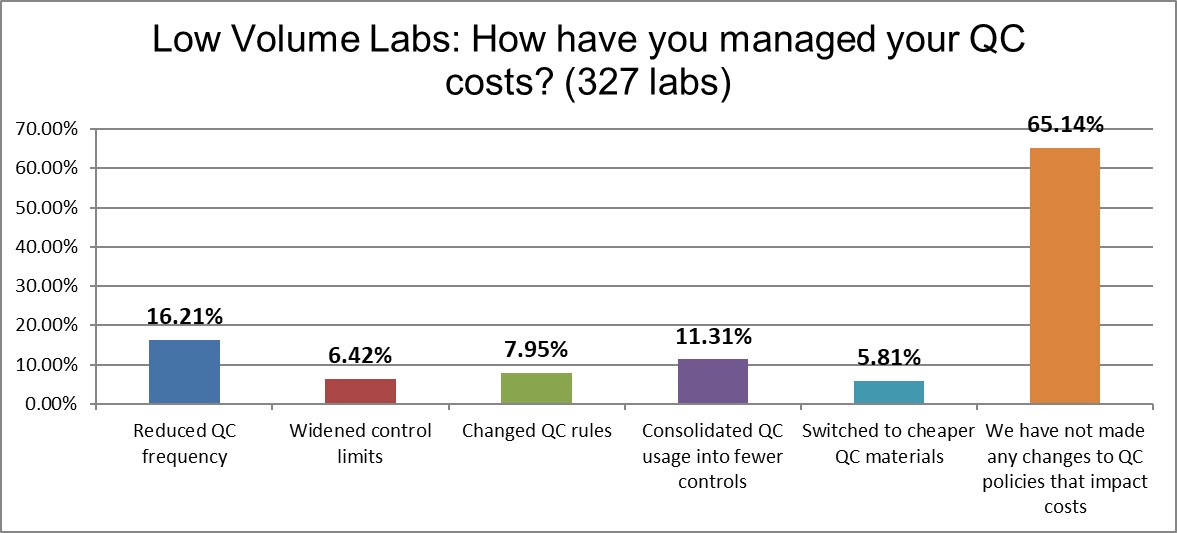
Almost two-thirds of both high and low volume laboratories have made no significant changes to their QC practices that impact their costs. Strikinginly, laboratories are very similar in their other QC improvement efforts. They have both reduced QC frequency, widened control limits, changed QC rules at about the same rate (although the lower volume laboratories are just slightly higher in all three activities). The biggest difference is that high volume laboratories have consolidated their QC into fewer controls (17% vs 11%).
If we began with an assumption that high volume laboratories must be doing something better in their QC than lower volume laboratories, the survey rejects that premise. There are more errors occurring and more repeating of controls occurring in high volume laboratories. The mega-laboratories may have achieved innovations in their scaling, logistics, and efficiency, but they do not stand out on the basis of how they practice QC.
Improvements are needed, regardless of the testing volume of a laboratory, and there is no one area we can point to as a shining example.