CGM quality, compromised?
A significant new paper just became available online, tackling the difficult challenge of CGM quality:
Clinical assessment and acceptance criteria for continuous glucose monitoring (CGM) system performance: A proposed guideline by the IFCC Working Group on CGM, Pleus S, Eichenlaub M, Dabla PK, Diem P, Boija EE, Fokkert M, Hinzmann R, Jendle J, Klonoff DC, Lu J, Makris K, Mohan V, Nichols JH, Pemberton JS, Selvin E, Slingerland RJ, Thomas A, Tran NK, Witthauer, Freckmann G. Clinica Chimica Acta 580 (2026) 120728
https://www.sciencedirect.com/science/article/pii/S0009898125006072
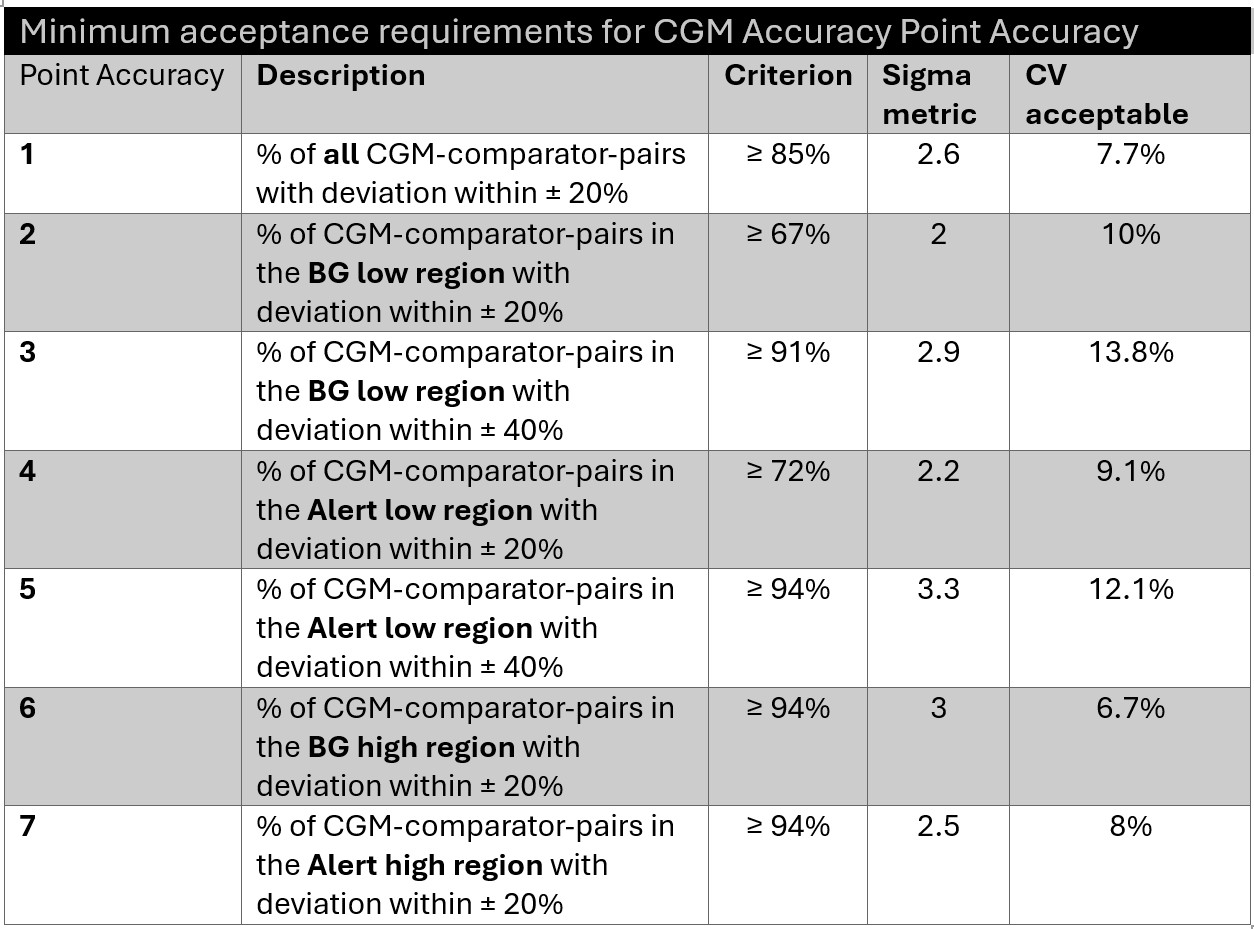
In the paper, the committee proposes 7 different minimum point accuracy requirements, for different ranges of the method. By proposing not only the goal, but also the % success at achieving that goal, the committee is offering a glimpse into the Sigma metrics being required of CGMs. (See the table below)
For example, if you ask for 85% success (a 15% defect rate) for an analytical goal of 20%, you're stating a 2.6 Sigma benchmark. If you're asking for 2.6 Sigma from a goal of 20%, you're also allowing 7.7% imprecision (in the absence of any bias).
It turns out most of these minimum requirements are below 3 Sigma, which is generally considered the minimum acceptable performance in other spheres of operations. We demand far higher performance of our core laboratory methods, even our POC devices.
Typical of this age, our "advances" appear to be coupled with diminished expectations. #cgm #glucose #diabetes #quality
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.
Comments