Basic QC Practices
The 2021 European (and others) QC Survey Results
Europe, Canada, Australia, the "Developed" world. If we look at all the advanced healthcare systems - and rule out the US because it's such a bizarre outlier of how to run a healthcare system and the CLIA regulations are equally unusual - what are the "best" labs doing with their QC?.
The 2021 Europe (and Canada and Australia) QC Survey Results
Sten Westgard, MS
November 2021
[This survey was completed with the support and partnership of Technopath Clinical Diagnostics.]
- The Top Ten Findings of the 2021 Global QC Survey
- The 2021 Global QC Survey
- The 2021 US QC Survey Results
- The 2021 Asia QC Survey Results
- The 2021 Middle-East QC Survey Results
- The 2021 Latin and South America QC Survey Results
- The 2021 European QC Survey Results
When we look at the world of QC, we can't leave out Europe. Are the "most developed" healthcare systems in the world also running the most advanced QC implementations? Let's see what this "developed" world is doing.
[Note that we're lumping Canada and Australia into Europe (which for Australia is a bit of double-counting, since we also included them in Asia.)]
The demographics
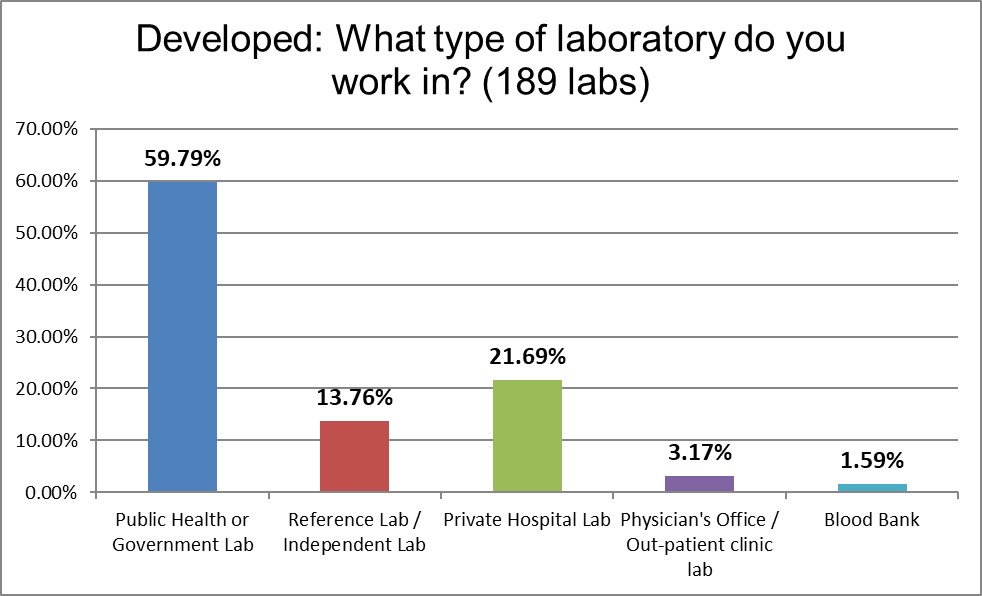
Public hospitals dominated our Developed respondents. Not surprising, since socialized healthcare dominates this region.
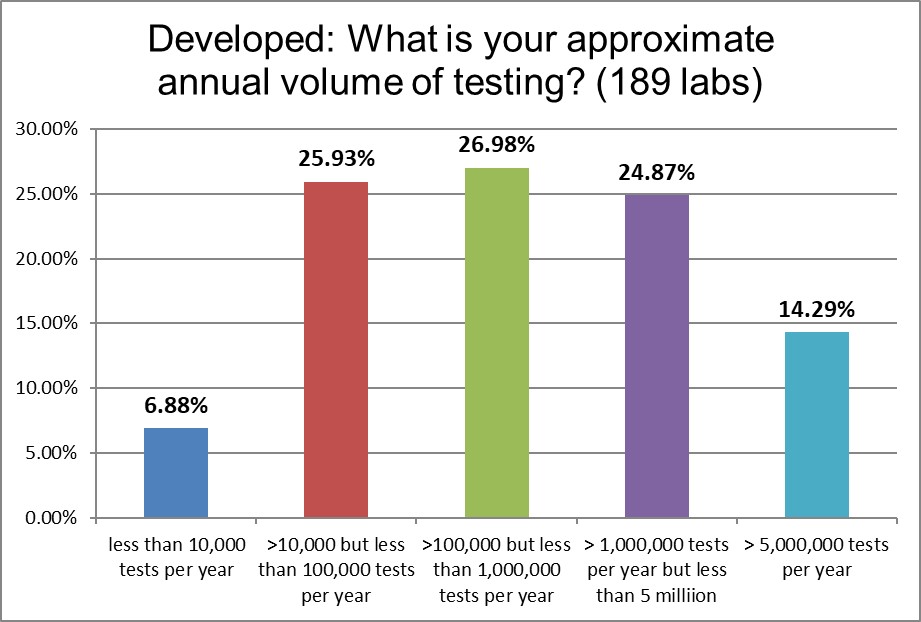
Very few small labs from this region participated in this study. There are significantly more large and "mega" laboratories in this group.
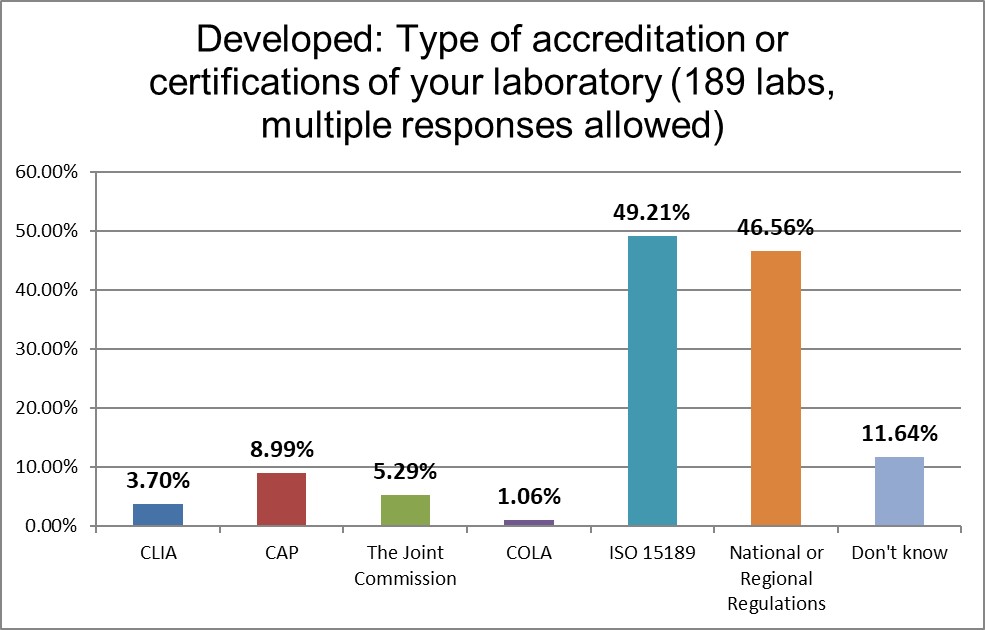
ISO 15189 and other local regulations are the determining factor, while CLIA and CAP has very, very little use in these countries.
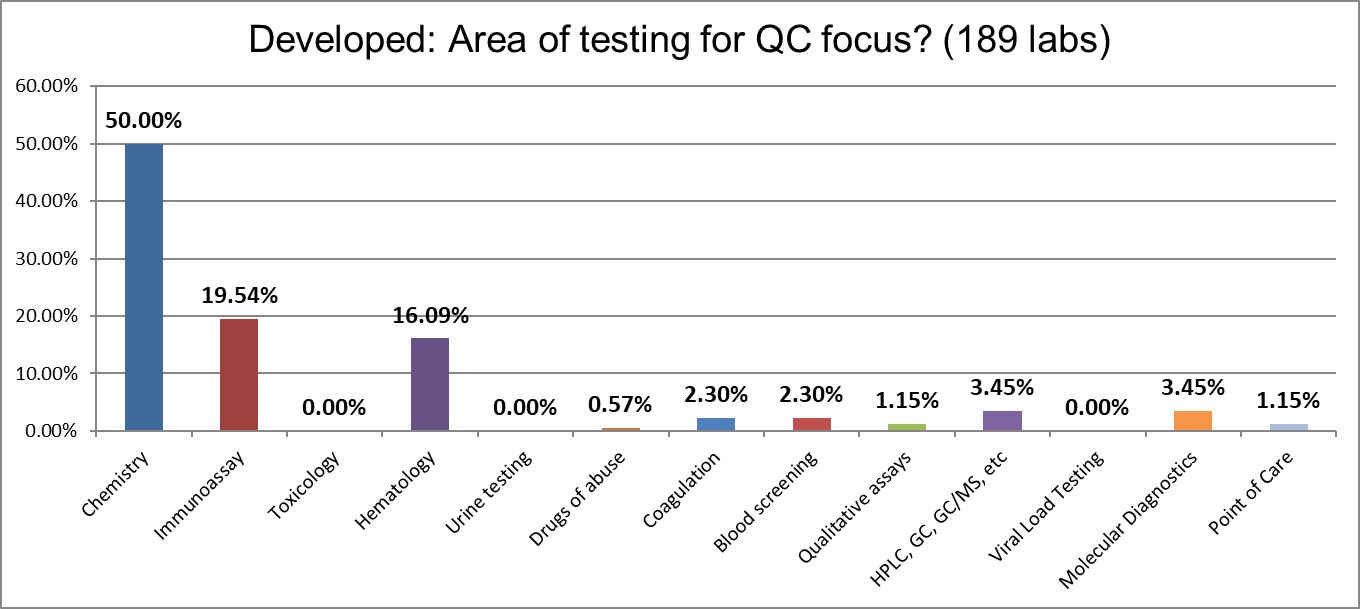
The respondents provided the most diverse set of responses, with a strong showing of hematology and immunoassay testing.
Now, how do these labs practice QC?
The QC Set Up
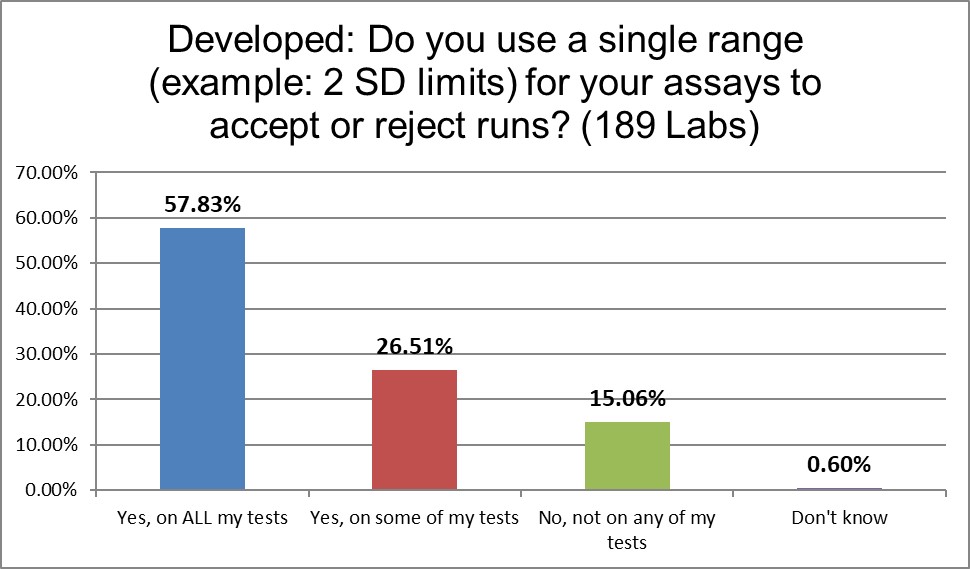
Over 80% of lab0ratories are using 2 SD control limits.
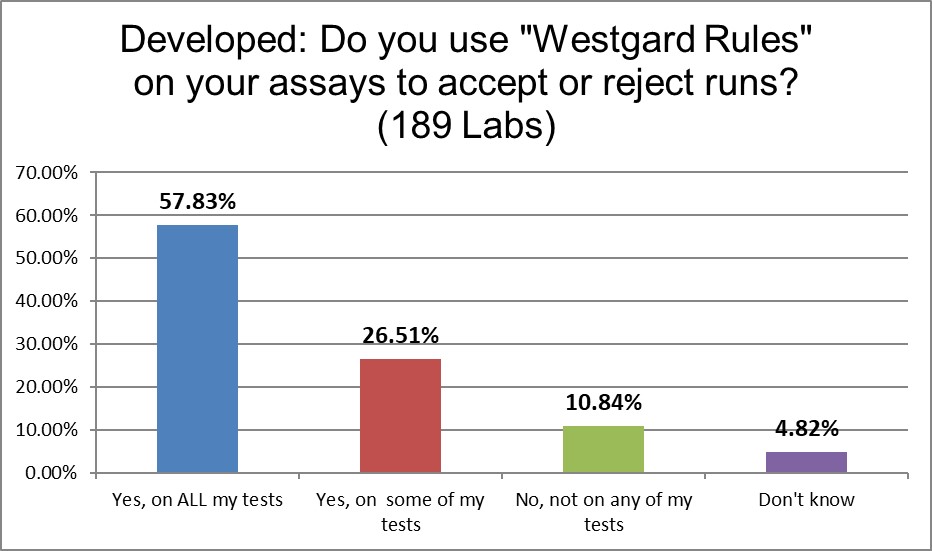
Amazingly, laboratories are using "Westgard Rules" at the same rate as they use 2 SD. (The US actually uses the "Westgard Rules" less than Europe.)
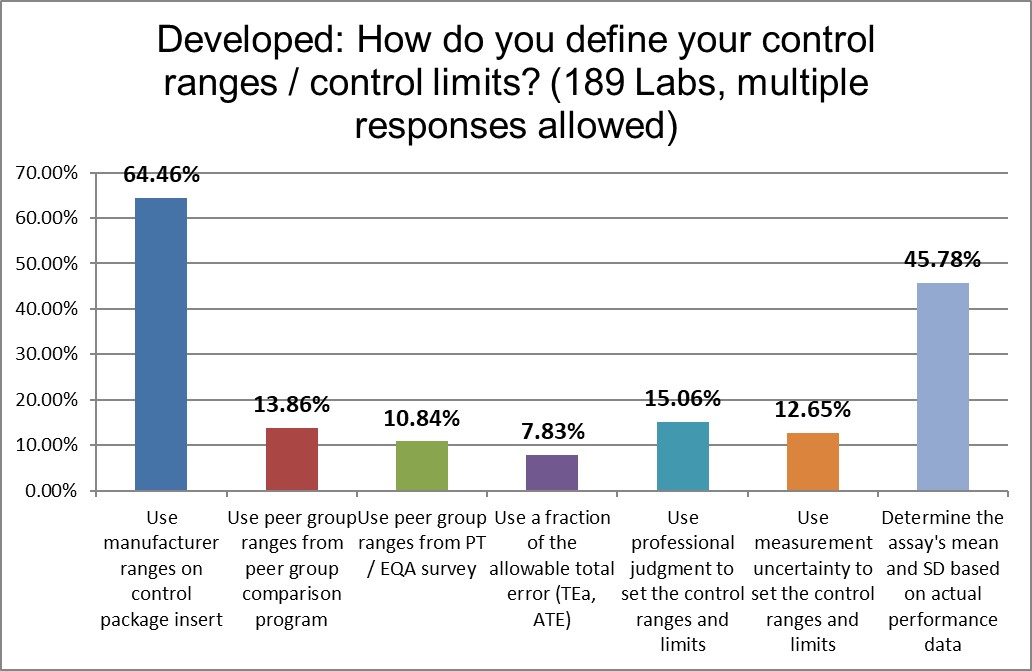
Surprisingly, less than half of laboratories are establishing their own mean and SD, and nearly two-thirds of laboratories are using the manufacturer ranges. Further one in eight labs are using measurement uncertainty to set their ranges. There are a lot of different ways to set ranges, and it appears that laboratories in the developed world are exploring most of them.
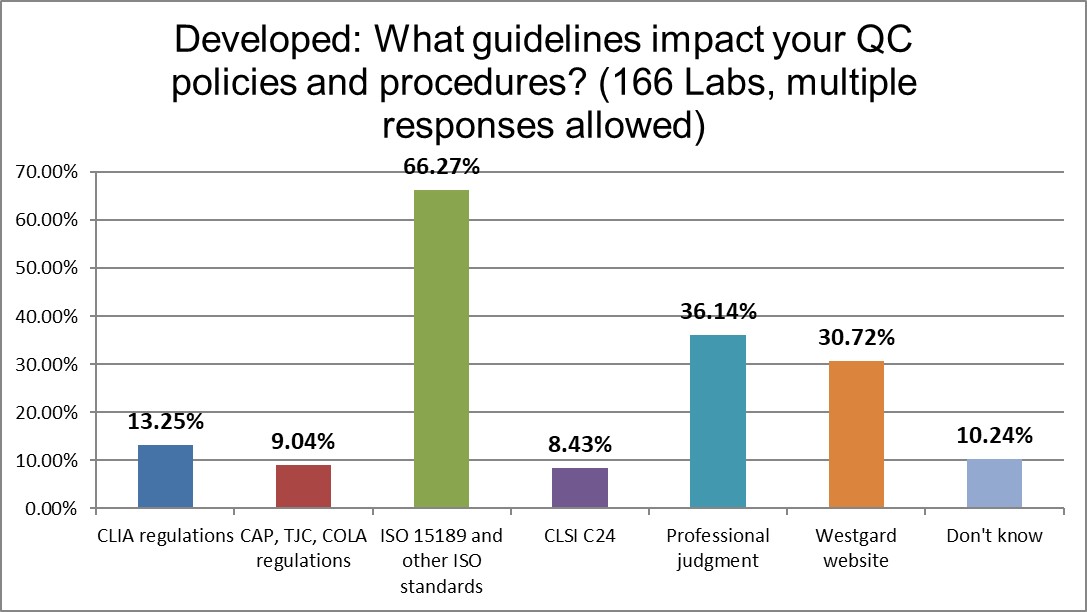
ISO 15189 dominates the regulatory guidance for QC, followed by professional judgment. Indeed, the highest use of professional Judgment occurs in the Developed region than anywhere else in the world. QC seems to be an area where laboratories feel their more free and individual. The Westgard website is significantly more influential than CAP and CLIA regulations.
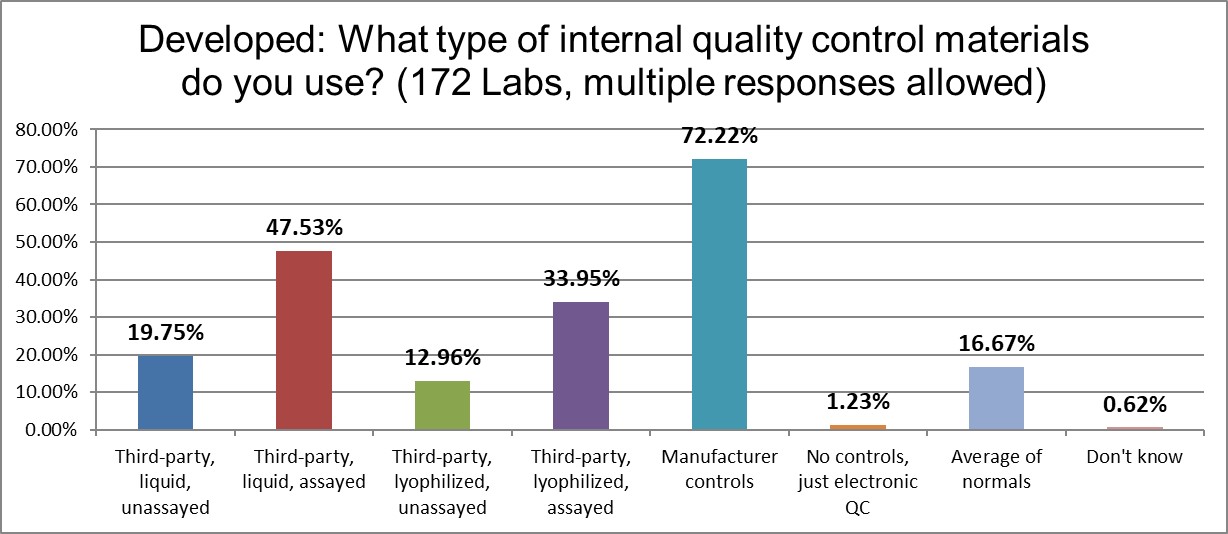
The use of manufacturer controls is the highest rate in the world. Third-party assayed lyophilized controls are used here nearly twice as much as in the US. Third-party liquied unassayed controls are used at half the rate as the US. It's a significantly different control marketplace.
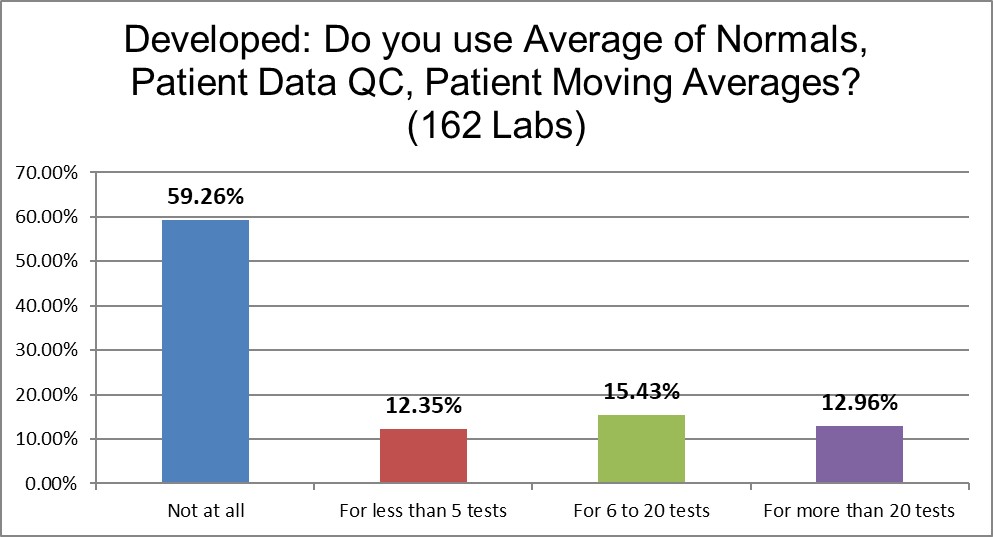
Almost 40% of laboratories are using some form of PBRTQC. Similar to the range setting, the Developed world is at the cutting edge of using alternative QC techniques. They use these twice as more often as the US.
The Real Practice of Running Controls
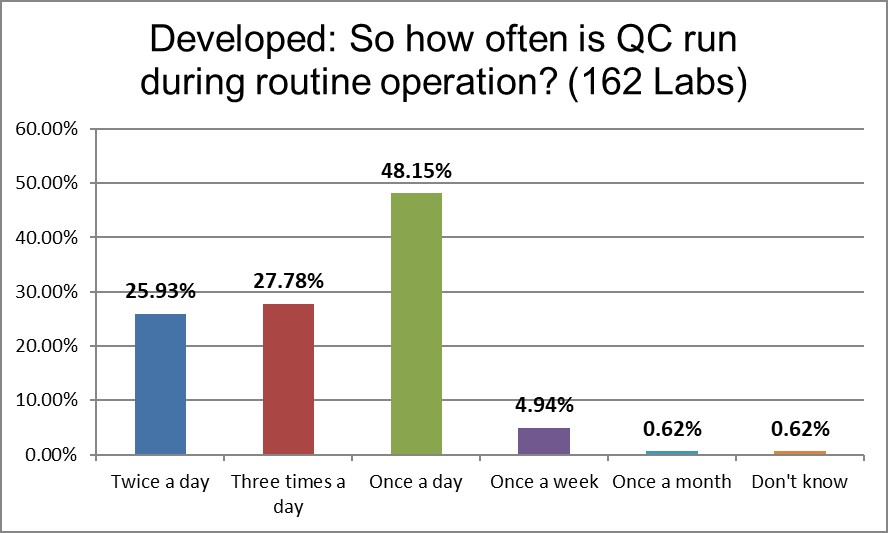
More labs are running controls 2 or 3 times a day than running QC once a day. This is highest use of controls in the world.
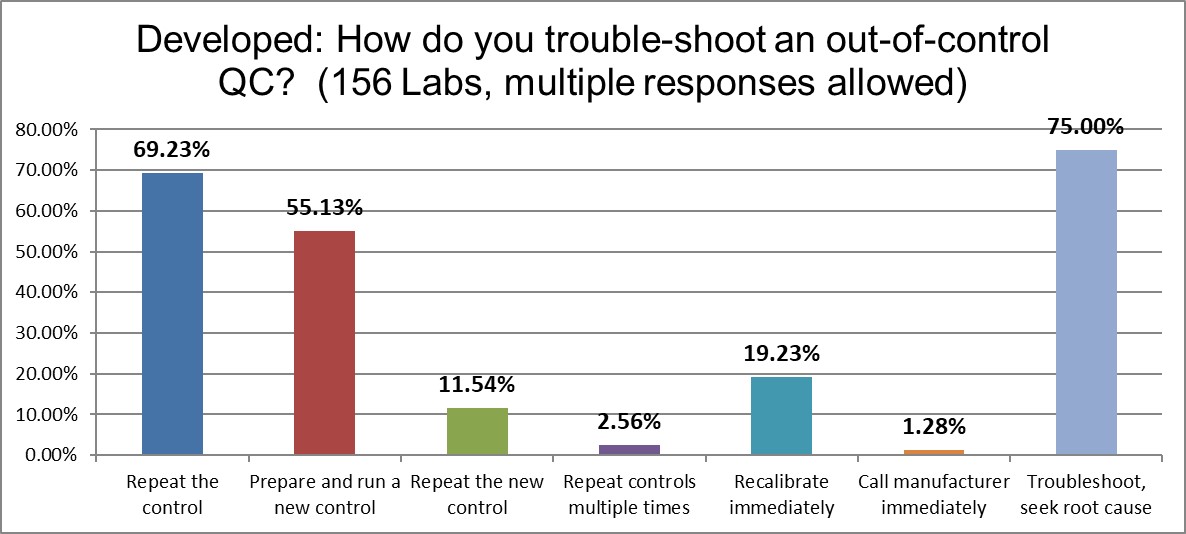
Three-quarters of labs in the Developed world troubleshoot and seek root cause, but almost 70% repeat the control when it's out. The US still leads the world in repeating and running new controls, but this region is second. Control vendors must be rather happy to see the biggest marketplaces are also the ones with so much repeating of controls.
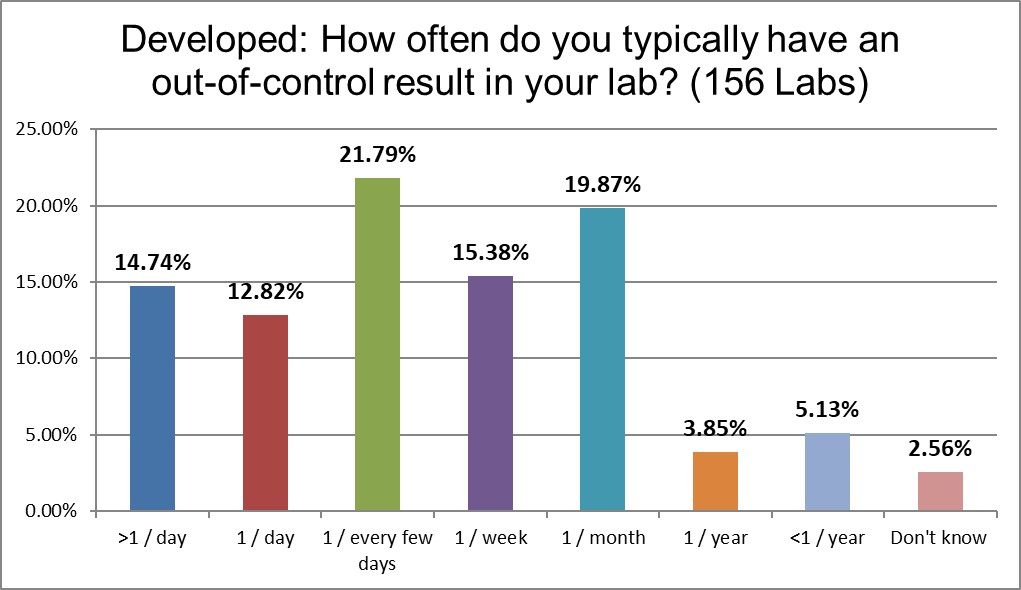
A quarter of laboratories report being out of control once a day or more than once a day, similar to the US rate. But a third of labs are out of control only once a week or once a month, significantly better than the rest of the world.
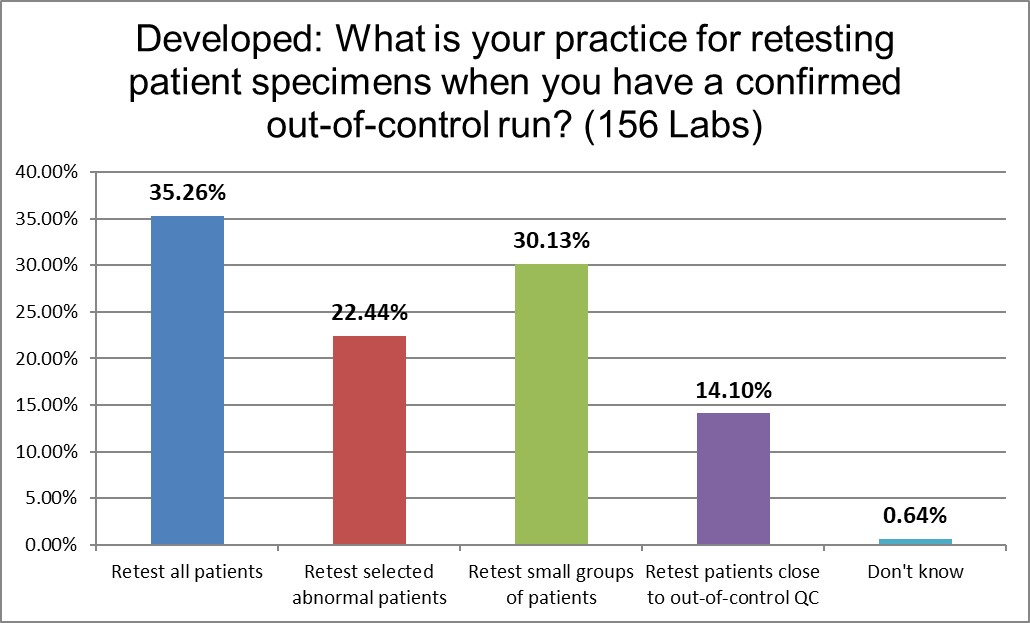
The Developed laboratories Retest all patient specimens at a slight higher rate than the US, and retests patients with abnormal values significantly more than the US. Otherwise, very similar.
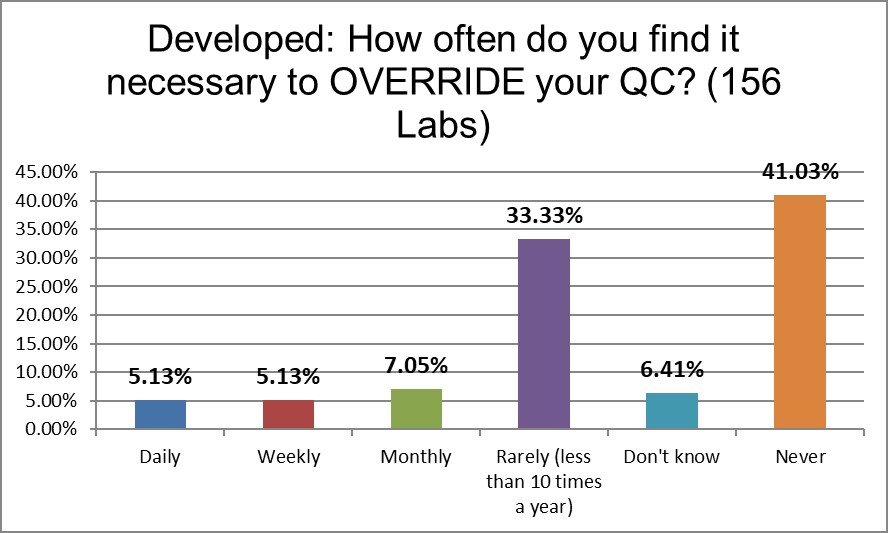
Labs in Europe et al are more than five times more likely to override their QC on a regular basis than the US.
The Final Overview
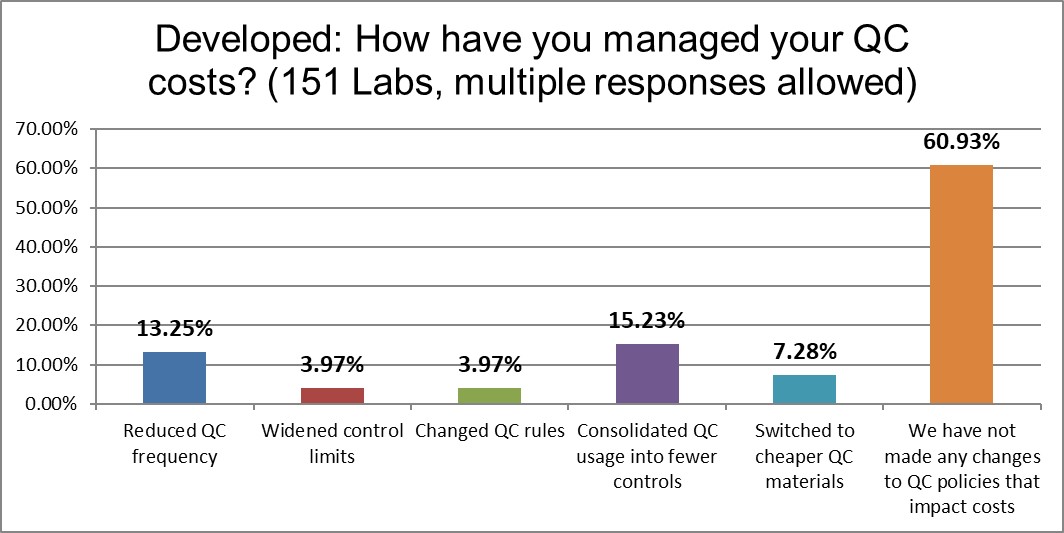
While there are lots of highs and lows, the Developed labs have done less to manage their QC costs than the global average (in terms of reduced QC frequency, wider control limits, and QC rule changes). The US has done less still, but the practices in Europe et al are remarkably stagnant. Given the vast amount of QC being performed in this region, there is clearly more work to be done in optimizing practices.
