Sigma Verification of Performance Program
Laboratorio de Analisis Clinicas (LAC) of Montevideo, Uruguay Sigma Verification of Performance
LAC - Laboratorio de Analisis Clinicos - of Montevideo becomes not only the first Uruguayan Sigma VP lab, but the first laboratory in all of South America to receive the Sigma Verification of Performance.
Sigma Verification of Laboratorio de Analisis Clinicos
Verification: September 14, 2018
Duration of Verification: September 14, 2018 through September 30, 2019

"In February 1999, the LAC opened its headquarters of the Pan American Building. From the beginning it formed a compact technical group, it was strongly oriented towards automation and control in bases to computer tools; concomitantly developed and demonstrated its strong commitment to institutions, doctors and their patients.
"In 2007 he inaugurated his new plant, adapting the building conditions of the old radiological clinic of Dr. García Capurro, which have allowed him to develop his activities in an environment according to his needs.
"These years of work, effort and dedication position us in the market as a laboratory of excellence.
"We carry out more than 760000 tests per year, we are the first accredited clinical analysis laboratory ISO 15189 in Uruguay, a partner of the Mayo Clinic Laboratories (MML - Mayo Medical Laboratories) and a member of ALADIL (Association of Latin American Laboratories). More than 80 institutions from all over the country request our services.
"Thanks to a team that works with professionalism and warmth, we manage to provide a service that provides the greatest security and confidence."

With Beatriz Varelaz and Gonzalo Pacheco, 2 of the Certified Quality Managers.
List of Assays Verfied at LAC
|
CHEMISTRY Assay |
Verified on Ci8200 44910 |
Verified on Ci8200 31286 |
|
Albumin |
Six Sigma |
Four Sigma |
|
Alk Phos |
Six Sigma |
Six Sigma |
|
Amylase |
Five Sigma |
Four Sigma |
|
ALT |
Six Sigma |
Six Sigma |
|
AST |
Six Sigma |
Four Sigma |
|
Bilirubin, Direct |
Six Sigma |
Six Sigma |
|
Bilirubin, Total |
Six Sigma |
Six Sigma |
|
Calcium |
Five Sigma |
|
|
Cholesterol, Total |
Six Sigma |
Six Sigma |
|
Creatinine |
Six Sigma |
Six Sigma |
|
Creatinine Kinase (CK) |
Four Sigma |
|
|
GGT |
Six Sigma |
Six Sigma |
|
Glucose |
Six Sigma |
Five Sigma |
|
HDL |
Six Sigma |
Six Sigma |
|
Iron |
Six Sigma |
|
|
LDH |
Six Sigma |
Six Sigma |
|
Phosphorous |
Four Sigma |
|
|
Potassium |
Six Sigma |
Six Sigma |
|
Protein, Total |
Six Sigma |
Six Sigma |
|
Sodium |
Four Sigma |
Four Sigma |
|
Triglycerides |
Six Sigma |
Six Sigma |
|
Urea |
Four Sigma |
Four Sigma |
|
Uric Acid |
Six Sigma |
Six Sigma |
|
Creatinine, Urine |
Six Sigma |
|
|
Total Protein, Urine |
Six Sigma |
IMMUNOASSAYS
|
IMMUNOASSAY Assay |
Verified on Ci8200 44910 |
|
CRP |
Six Sigma |
|
PSA |
Four Sigma |
|
T4 Free |
Four Sigma |
|
Transferrin |
Six Sigma |
|
TSH |
Five Sigma |

With Dr. Milton Fornella, Lab Director, signing the Verification Certificate.
Visual Display of Assay Performance at LAC
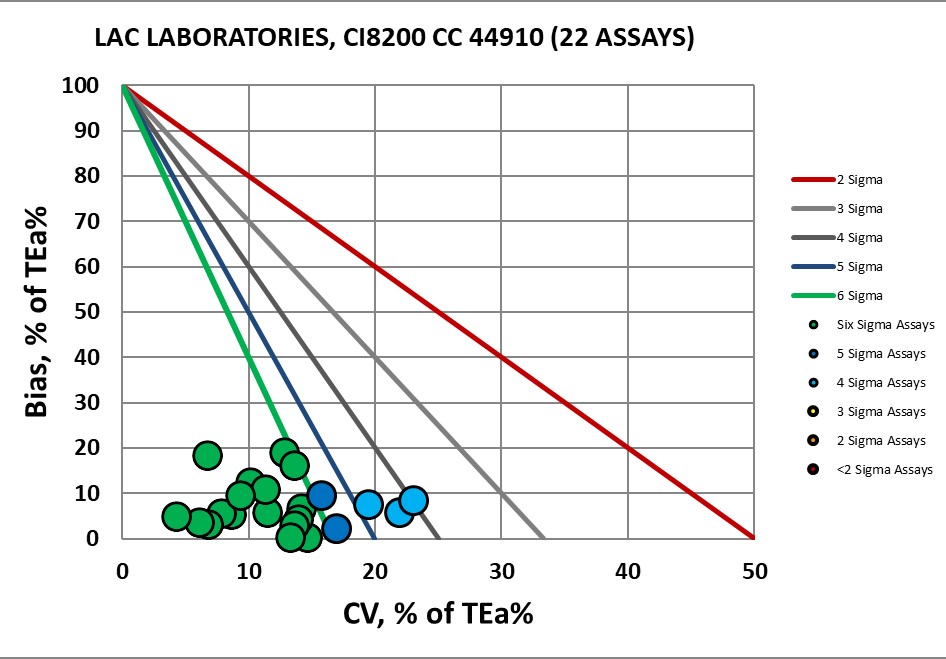
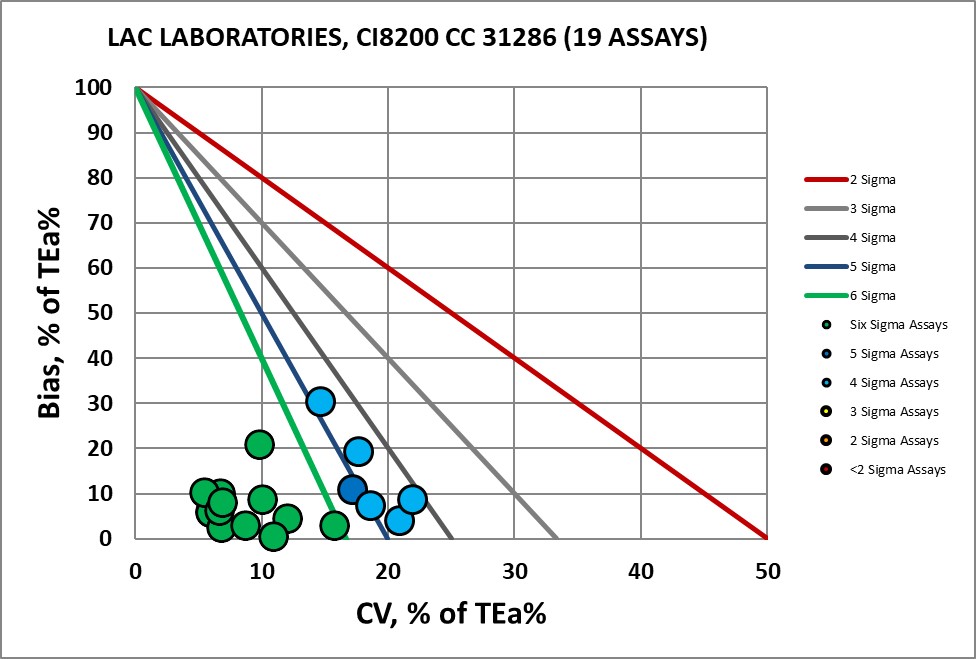
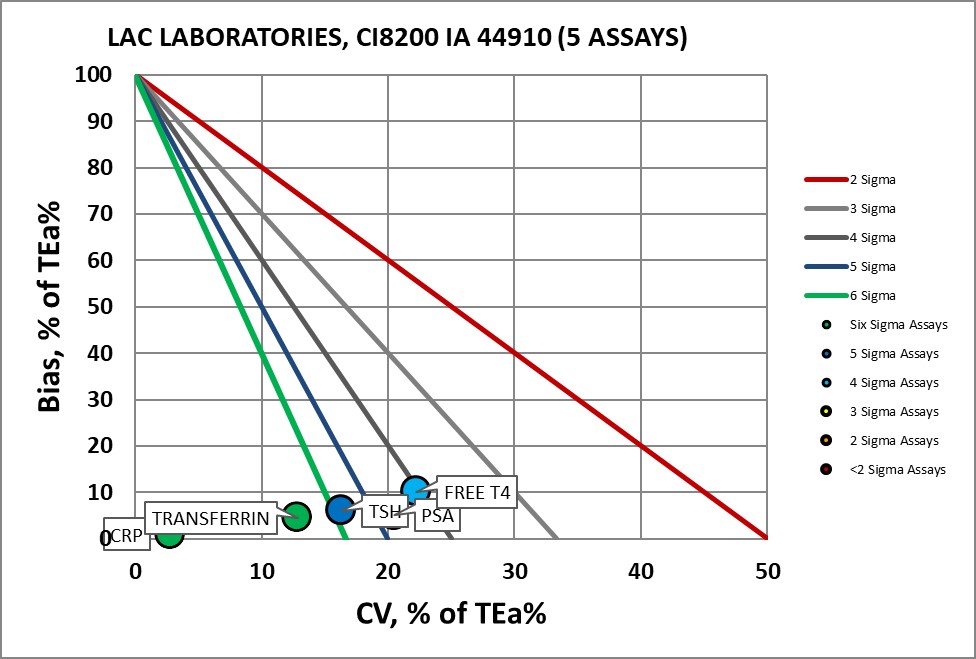
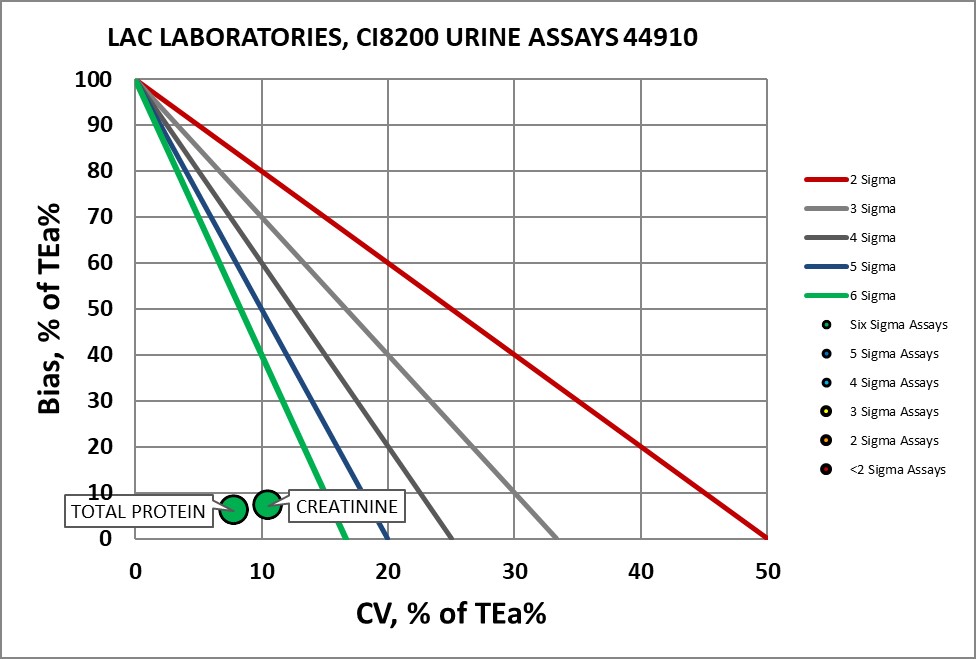

With Dr. Fornella, the completed Verification Certificate.
Congratulations to LAC for all the the excellent work they have completed.