Basic QC Practices
The 2021 Great Global QC Survey Results
The results are in! More than 600 laboratories from more than 100 countries around the globe answered the 2021 Westgard survey on QC practices. How are labs really performing quality control? The answers may surprise you.
The 2021 Global QC Survey Results Are "In" - and "Out"
Sten Westgard, MS
November 2021
[This survey was completed with the support and partnership of Technopath Clinical Diagnostics.]
- The Top Ten Findings of the 2021 Global QC Survey
- The 2021 Global QC Survey
- The 2021 US QC Survey Results
- The 2021 Asia QC Survey Results
- The 2021 Middle-East QC Survey Results
- The 2021 Latin and South America QC Survey Results
- The 2021 European QC Survey Results
It's 2021, have the practices of QC changed?
We surveyed laboratories back in 2017 about their quality control practices. We did it again in 2021.
The demographics of the participants
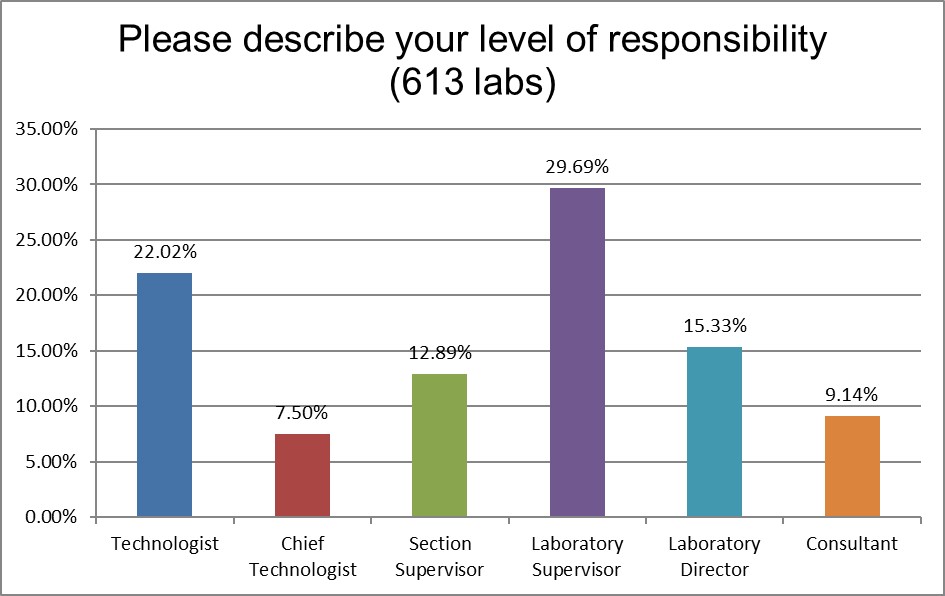
The majority of respondents are in direct contact with the bench, who directly implement the policies, not just direct others to do so.
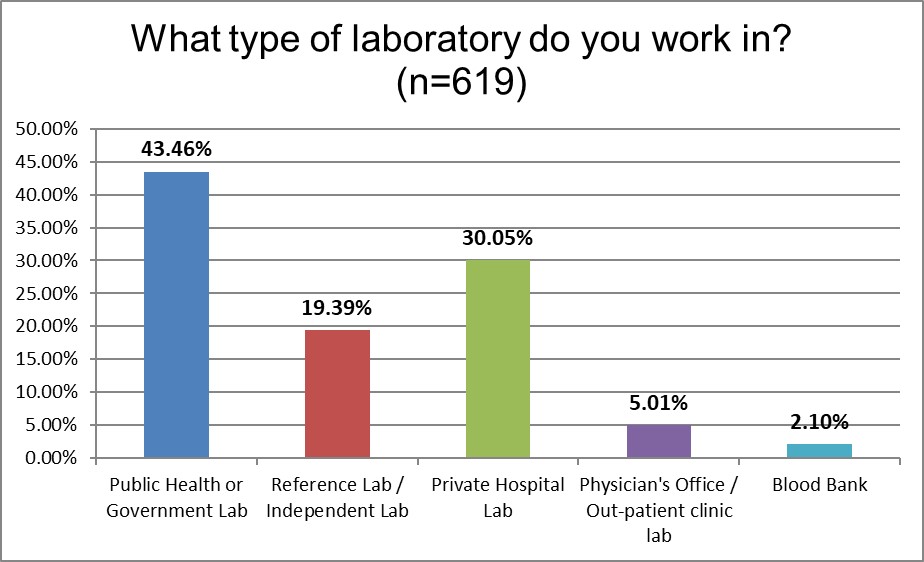
Since it is a global survey, it's not surprising that there are more public or government labs that participated in the survey.
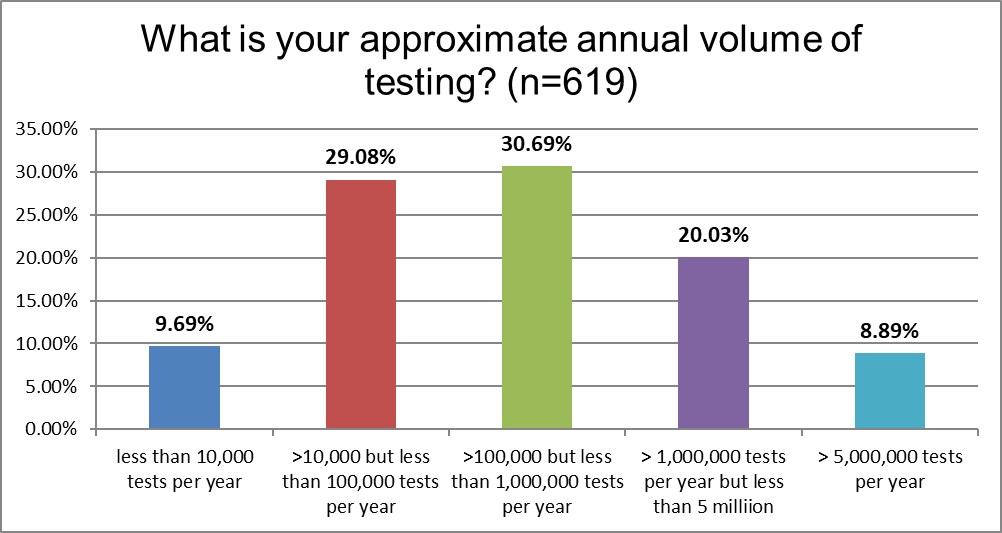
The tip of this bell curve is in the area of labs running 100,000 to 1,000,000 tests a year.
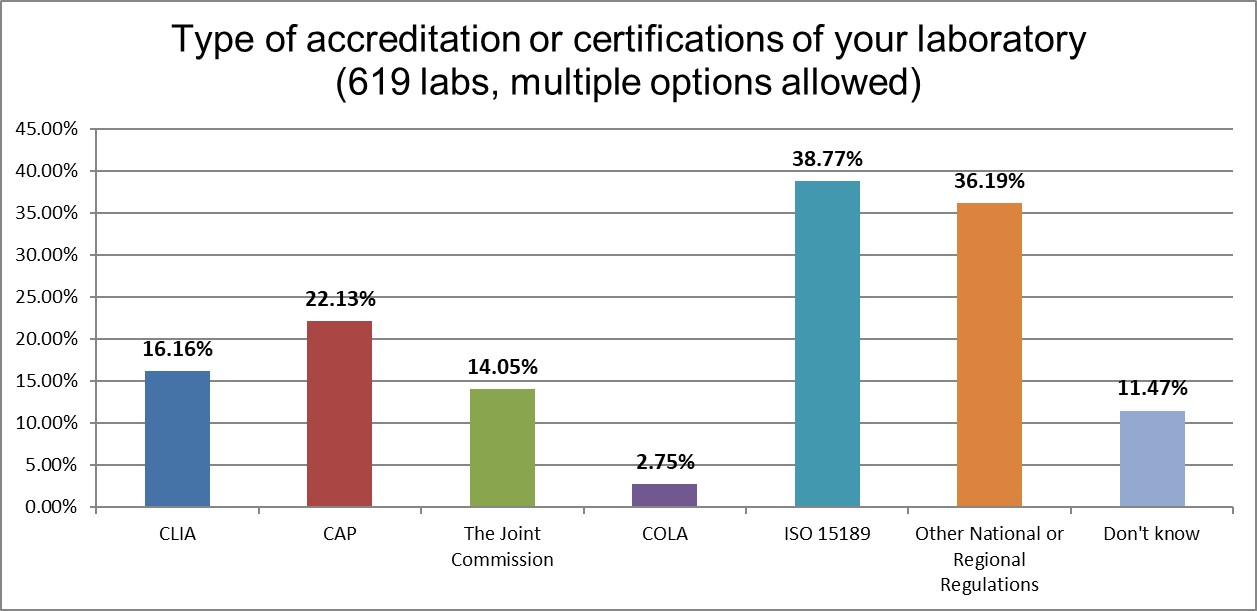
The ISO 15189 standard is the main regulation governing laboratories around the world.
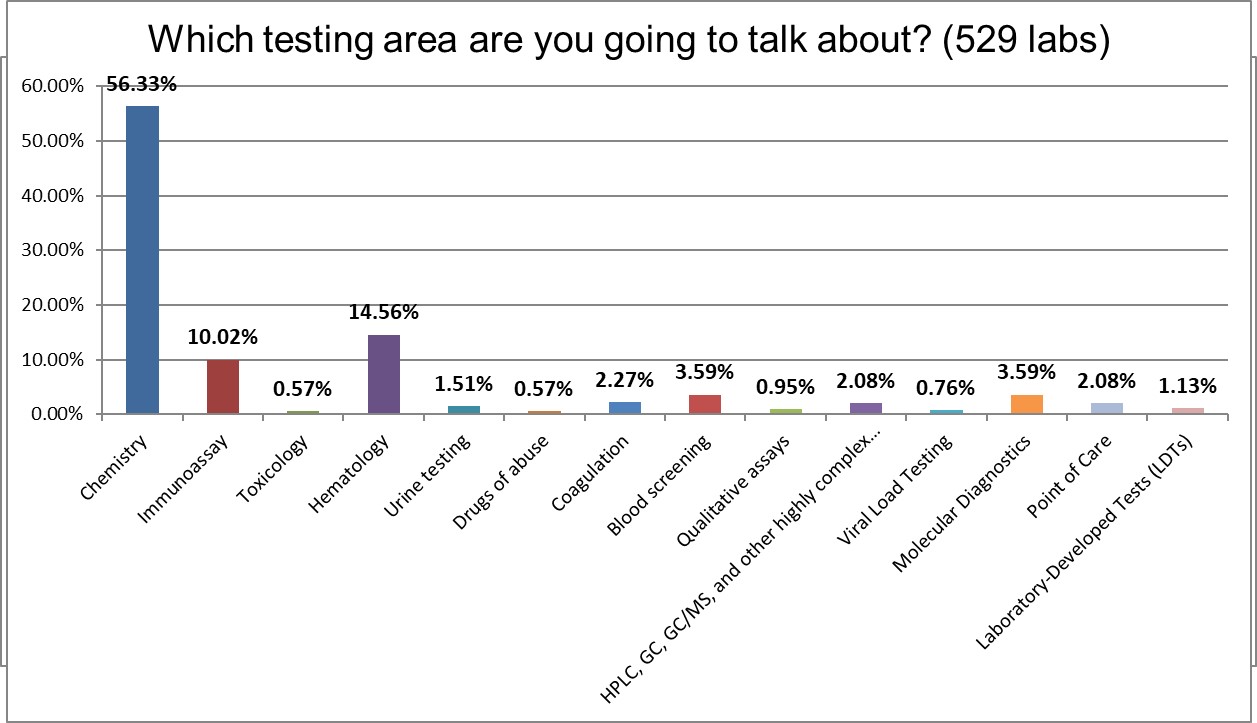
Participants were asked to focus on one area of testing in their responses, the predominant area of testing was biochemistry, followed by hematology, and immunoassays.
The QC Set Up
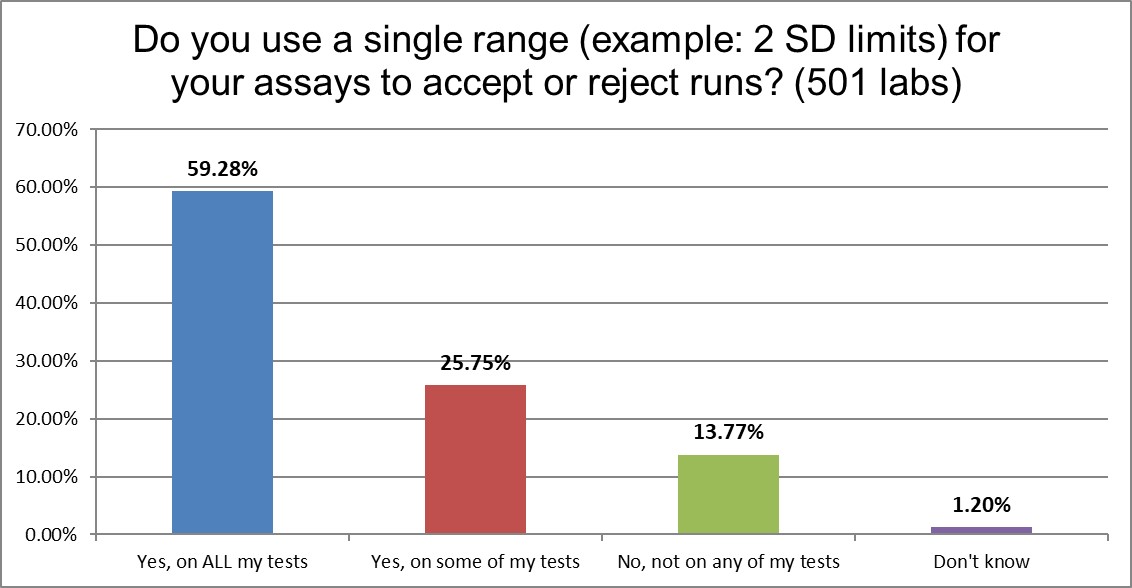
Most laboratories are still using 2 standard deviations as one of their primary QC techniques. This, despite the well-known false rejection rate caused by these limits.
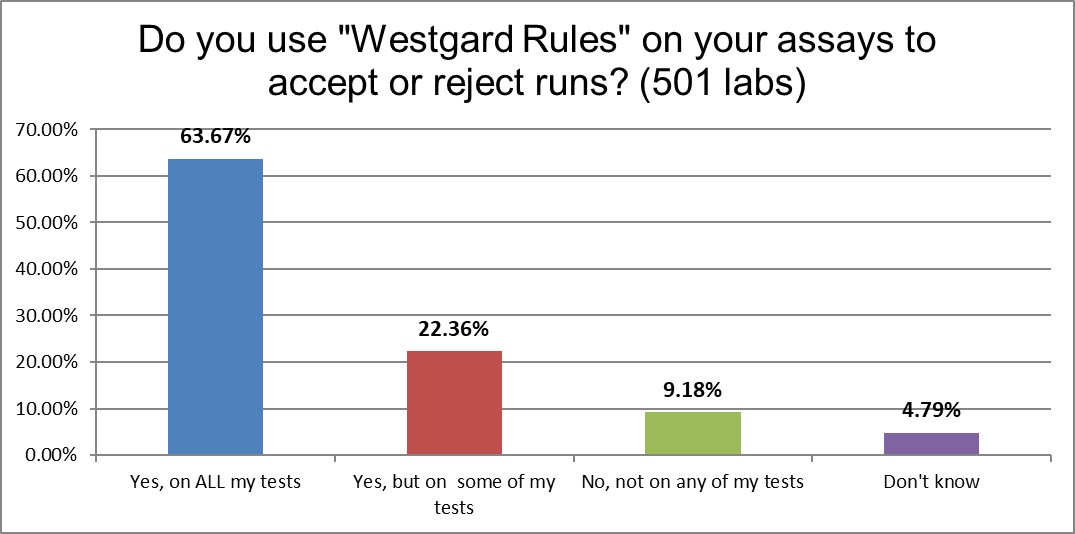
"Westgard Rules" are also still being used on most tests in a majority of laboratories, and are utilized to some extant in more than 86% of laboratories.
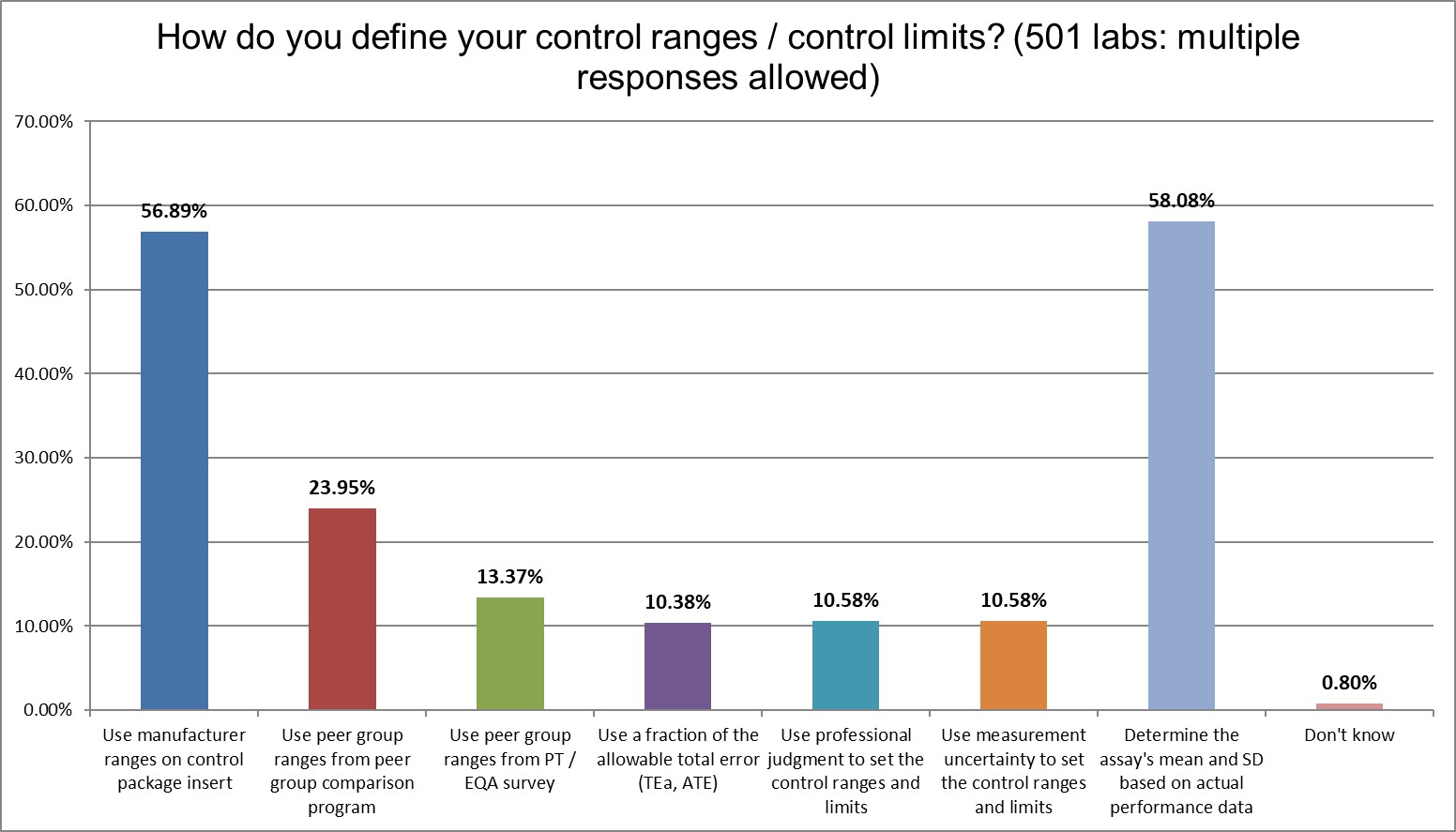
Labs know they should do the right thing, and the majority of them do so, calculating the actual mean and the actual SD to create their Levey-Jennings control charts. However, the second-most popular choice is also performed in the majority of laboratories: using a manufacturer's range to create their Levey-Jennings control chart. The former choice is best practiced, endorsed by regulations and the CLSI C24 guidance. The latter choice is convenient, easy, and usually generates control limits that are quite wide, therefore providing false comfort to the laboratory.
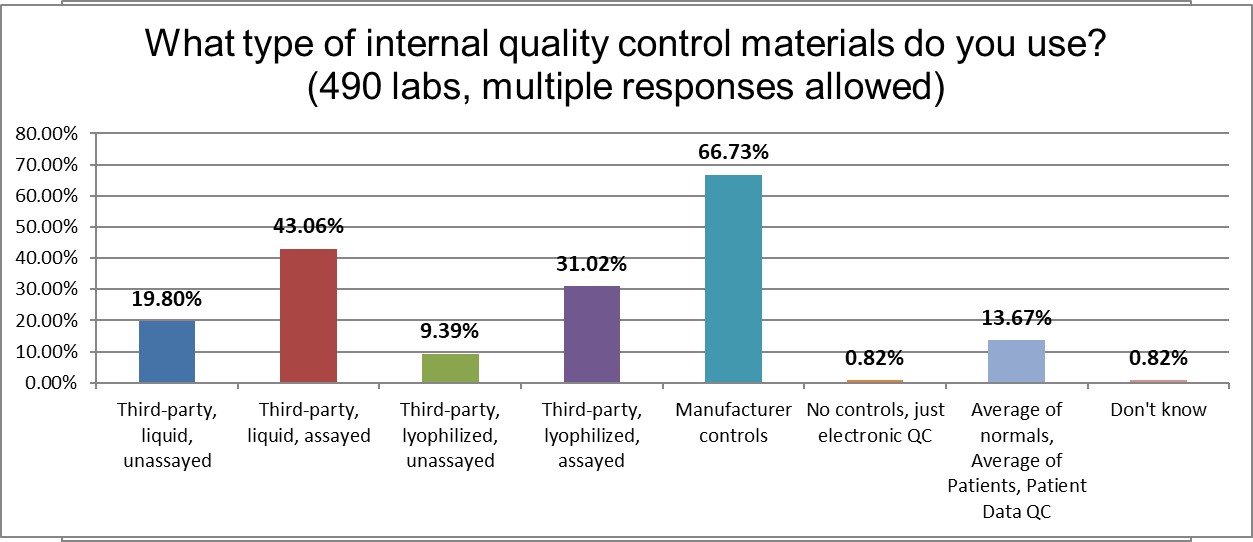
A majority of laboratories use the controls provided by manufacturers. The second-most popular control type is third-party, assayed, liquid controls. Lyophilized assayed control are the third most popular type of controls. Note that a little over 13% of labs state they use average of normals, average of patients, patiend data QC, the technique currently referred to by the in vogue term, PBRTQC (Patient Based Real Time QC).
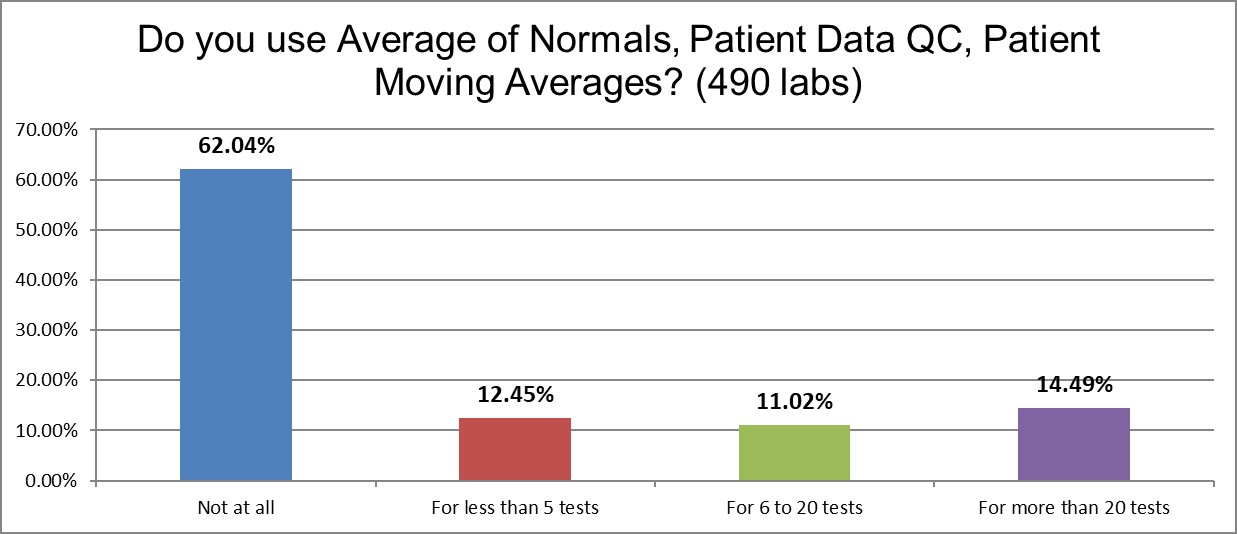
Given the heightened interest in PBRTQC techniques, we probed deeper. The answer is that more than 60% of labs are not using any average of normals techniques. About a fifth of labs are using it quite sparingly, only on a handful or a dozen of tests. About one of seven laboratories is using it on more than 20 tests.
The Real Practice of Running Controls
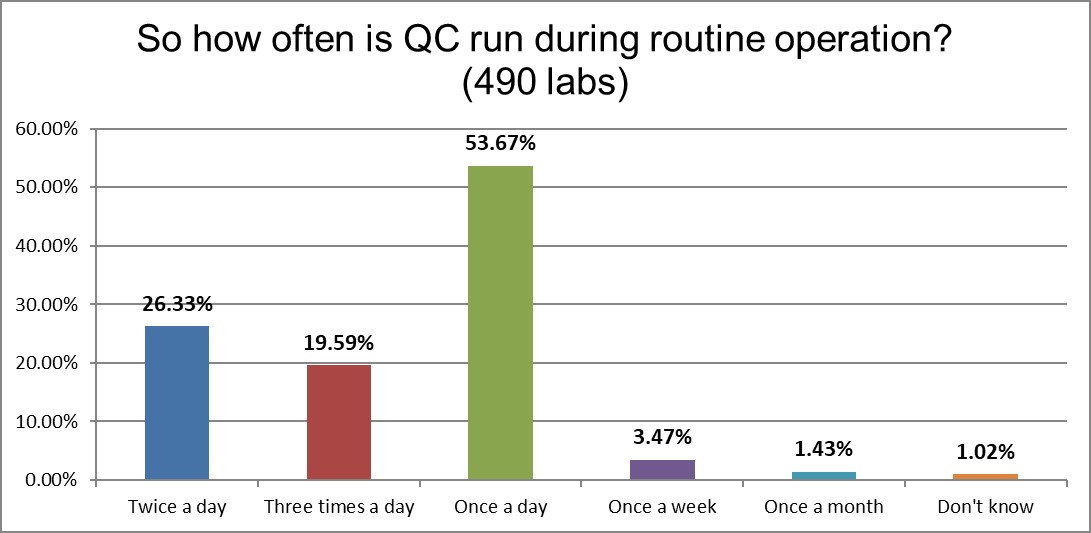
The standard of the world is once a day QC, with another quarter of the labs running twice a day, and another fifth running three times a day.
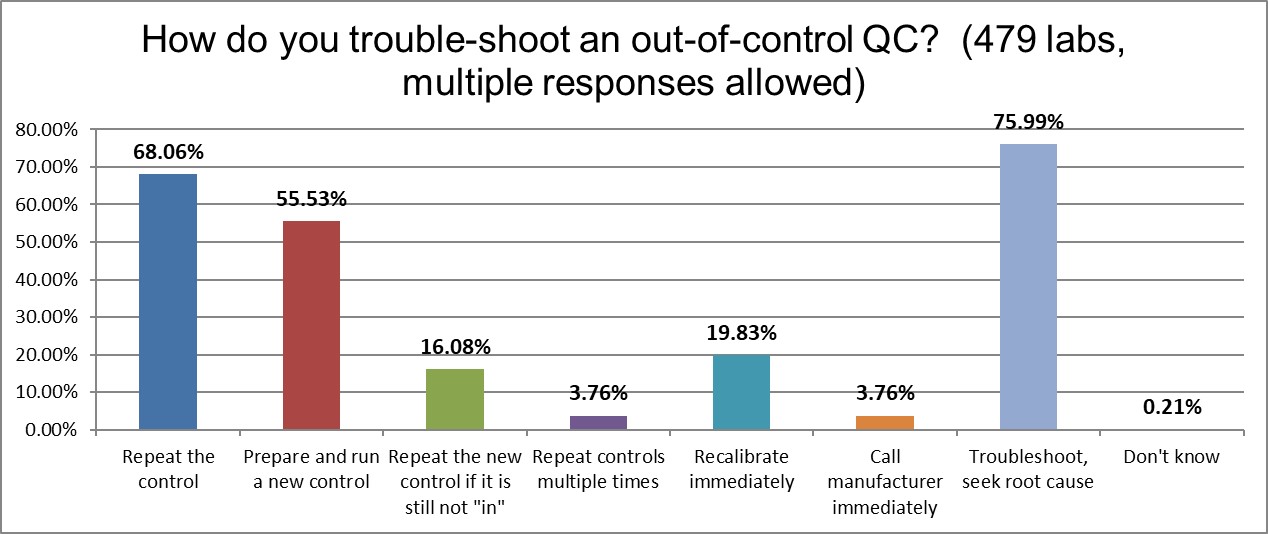
Three out of Four labs report that when they are out of control, they try to troubleshoot and seek root cause. But nearly as many, 68%, report that the just repeat the control. A majority will also run a new control. 16% will repeat the new control if it is not falling in. And almost 4% of labs were honest enough to tell us they will repeat controls multiple, multiple times in order to get the control back "in." This is a boon for control vendors, and a curse to laboratory productivity. Rolling the dice again and again on QC is not science, it's gaming the system.
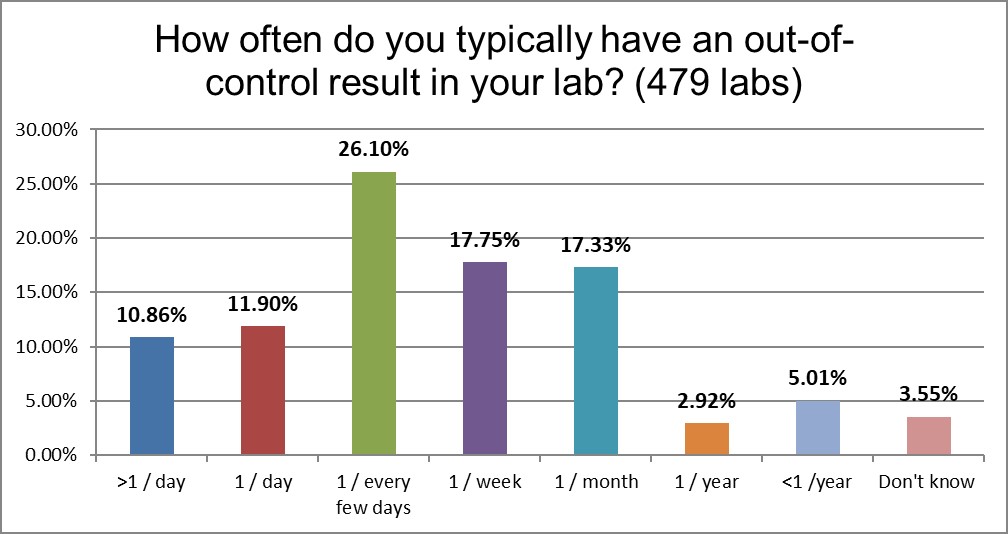
With all those repeats, you worry about how often these out-of-control events are occurring. Well, it happens to be fairly often. Close to a majority of labs experience failures every few days. And 1 in 5 labs experience a daily out-of-control event, if not more often. As we have said before, when the fire alarms are going off every day, the lab starts to lose its hearing.
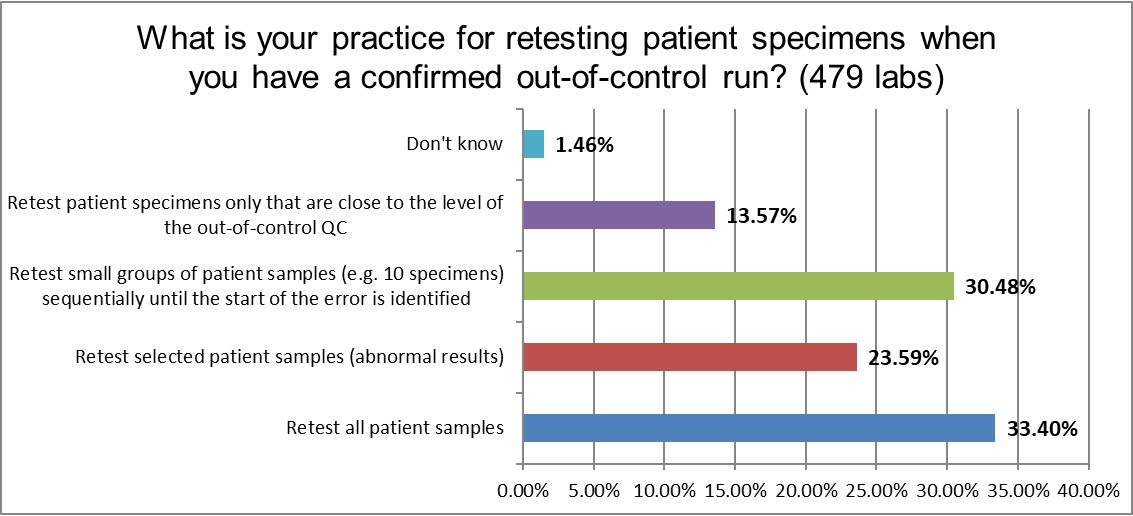
When a laboratory finally decides a run is out of control, how do they treat the patient specimens affected by the run failure? A third will repeat all the patient samples in the run, which what theory and textbooks tell them to do. A majority of labs are a little more selective, trying to repeat select patient specimens that would be more impacted by the failure, or trying to test specimens that are just around the "moment" of the error.
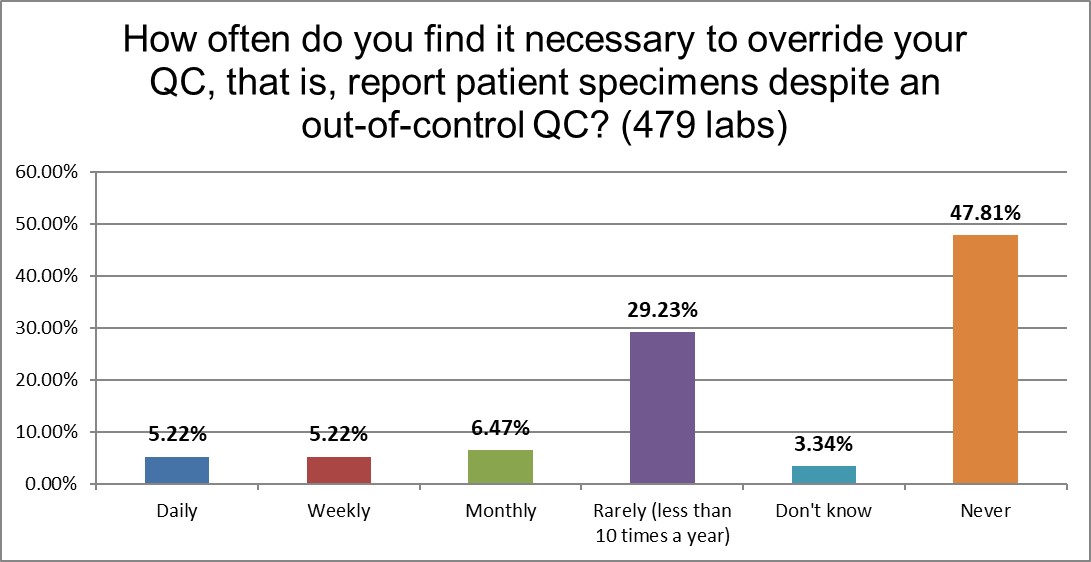
Given the pressure that laboratories face today, laboratories often feel like they have to release patient results, even when there is an out-of-control run. Almost 50% of labs, thankfully, do not ever do this. A little under a third do it rarely. But 1 in 10 labs are doing it weekly or daily.
The Final Overview
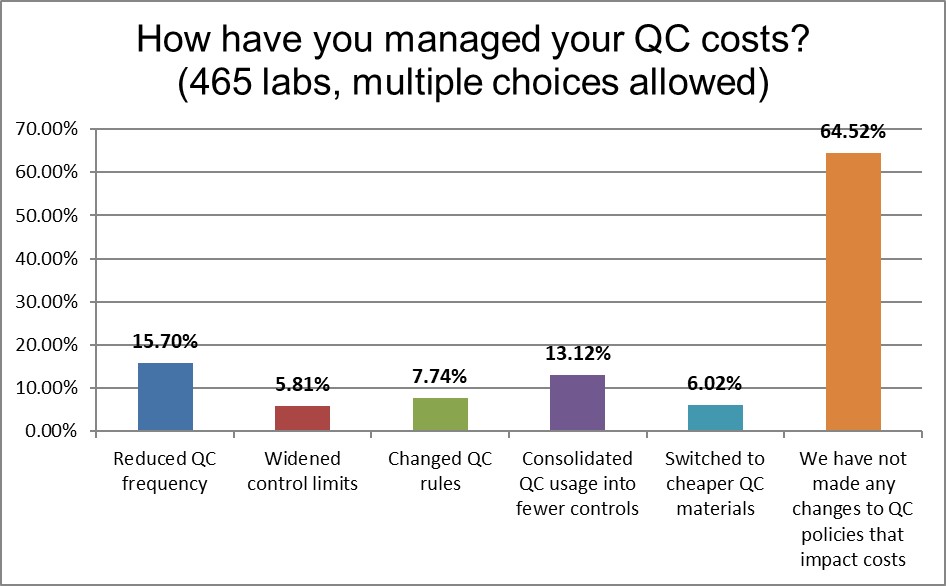
When we look over all the current practices, we see a lot of problems that can and should be fixed. However, almost two-thirds of laboratories have not made any changes to their QC practices. The most common action that labs have taken is reduced their QC frequency (which could be good if done for the right reason, and more dangerous if done for the wrong reason). The second most popular change that labs have been making is consolidating their QC into fewer controls, a prudent choice for the bottom line.
