Trends
LabQuality Days 2019: Reinventing Quality. Reimagining Control
I had the pleasure of attending the 2019 LabQuality Days in Helsinki. A few observations from my time there.
LabQuality Days 2019: Reinventing Quality? Reimagining Control
Sten Westgard, MS
February 2019

With thanks to Mia Lindstrom, Juha Wahlstedt, and Harri Laitinen
I had the pleasure of attending and presenting at "LabQuality Days" in Helsinki last week. I was very impressed by the caliber of the speakers at this event, a comprehensive roster of experts trying to reinvent how we do QC. There is clearly evolving idea of what we should consider acceptable and what should now be considered unacceptable. There was even an appearance by Dr. Curt Parvin, giving a historical perspective about the "MaxE(nuf)" model, which has now been harnessed to determine a data-driven QC frequency.

Dr. Curt Parvin, explaining how he came up with the MaxE(nuf) model
If I might mention some other highlights:
- Marc Thelen, of SKML, explaining why their EQA program offers two different Sigma-metrics. One Sigma-metric is based on the "Ricos goals", while another is based on a "state of the art" - for when no instrument can achieve the "Ricos goal". Succinctly: the laboratory shouldn't be punished for the failure of the manufacturer. This echoes one of the conclusions of the Milan Consensus, that some tests will use different models than other tests. There is no "one right answer" of analytical performance specifications for all tests and all labs. It implies there is a bigger challenge for us to tackle: which tests will need to use different models? And what about the difference in performance between European laboratories and elsewhere in the world? Will that mean we have to have different goals for labs in Africa, the Gulf, India, Southeast Asia, etc?
- Tony Badrick, of nearly everything in Australia, suggesting that IQC is too imperfect, while using patient samples for QC is a much more commutable, viable, and reliable way to monitor method performance. The idea that we no longer need internal controls is quite a suggestion indeed. The Average of Normals and other Patient Data QC techniques have been with us for decades, but the challenge has not been the theory, but the implementation. A robust set of informatics needs to be in place to set up the filters to exclude certain "abnormal" patient samples while accumulating the "normals" and calculating a good mean, median, etc. Dr. Badrick showed there are ways that these techniques can be effectively deployed to laboratories. Of course, It's hard to see how all the regulatory systems will respond to this major change - I think running controls is one of the easiest "compliance" activities. The other major obstacle is that many laboraties that don't have the informatics capability to implement Average of Normals techniques. When we have done surveys of global practices, we see around 10% of labs actively trying to use Average of Normals. That's a big mountain to climb if we really want to make Bio-Rad extinct.
- Hassan Bayat, our friend and colleague made a presentation of the MaxE(nuf) model. His ability to explain a very complex mathematical model is unique to our world. Neither I nor my father, possibly not even Curt Parvin himself, could explain it better! One revelation he shared this time is that if a method achieves 7 Sigma, by the Parvin model, there only needs to be one control run every 56,000 patient samples!! That's pretty reliable performance!!
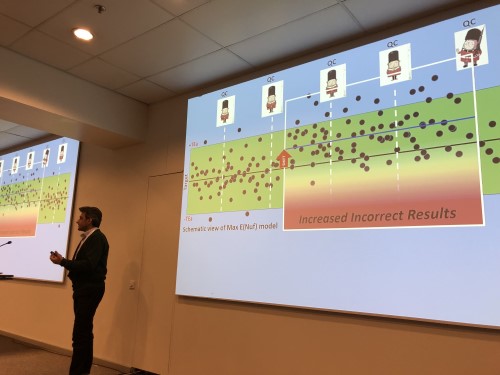
Dr. Hassan Bayat, showing an example of increased risk to patient results.

Our great friend Oswald Sonntag, to whom I award an unofficial "best audience question" award, for asking the expert panel, "Why are we here?"

With Luis Valenzuela Andrade, my colleague from Chile, at the LabQuality "Q"

Thanks again to LabQuality and the conference speakers and attendees. It's a memory I will not easily forget.