"Westgard Rules"
Westgard Sigma Rules
We've talked about Westgard Rules for decades, and we've talked about Six Sigma for years. So what happens when we try to talk about both at the same time? Westgard Sigma Rules
GAMECHANGE: AN ON-DEMAND WEBINAR ON CLIA
Introducing Westgard Sigma RulesTM
James O. Westgard, Sten A. Westgard
September 2014
We have long advocated customizing Statistical QC procedures, such as “Westgard Rules”, to take into account the quality required for the intended use of a test and the imprecision and bias observed for a method. Over time, we have developed a variety of QC design and planning tools to support laboratory efforts to select SQC procedures that are right for their specific intended clinical use and the method performance observed in their laboratory.
This work began many years ago with the development of a computer simulation program that could be used to determine the rejection characteristics of various control rules and different numbers of control measurements [1]. The tools include power function graphs [2], critical-error graphs [3], QC Selection Grids [4], charts of operating specifications (OPSpecs chart) [5], and the QC Validator [6] and EZ Rules 3 computer programs [7]. We continue to look for faster and simpler tools that will help laboratories select the right SQC for their own applications.
In our recent book Basic Quality Management Systems [8], we introduced a new tool that is quicker and easier to use than previous tools. We call this tool “Westgard Sigma RulesTM” to distinguish this approach from the original Westgard Rules.
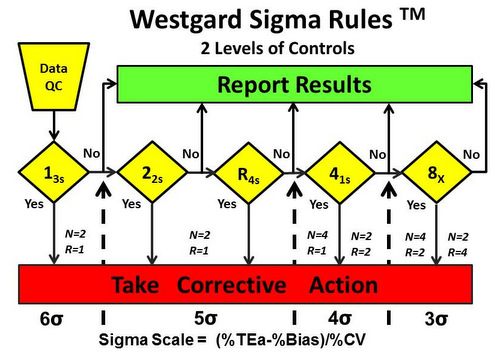
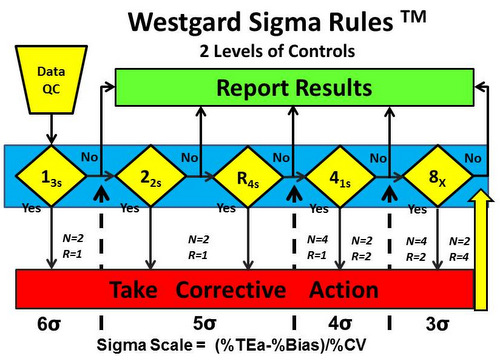
Figure 1 shows the Westgard Sigma Rules for 2 levels of control materials.
On first glance, it looks just like the Westgard Rules diagram except there is no 2 SD warning rule. That is an important distinction, but the most important change is the Sigma-scale at the bottom of the diagram. That scale provides guidance for which rules should be applied based on the sigma quality determined in your laboratory.
Here’s how it works. The dashed vertical lines that come up from the Sigma Scale show which rules should be applied based on the sigma quality determined in your laboratory. For example:
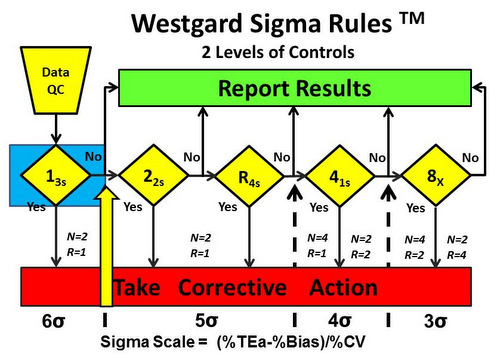
- 6-sigma quality requires only a single control rule, 13s, with 2 control measurements in each run one on each level of control). The notation N=2 R=1 indicates that 2 control measurements are needed in a single run. See Figure 2.
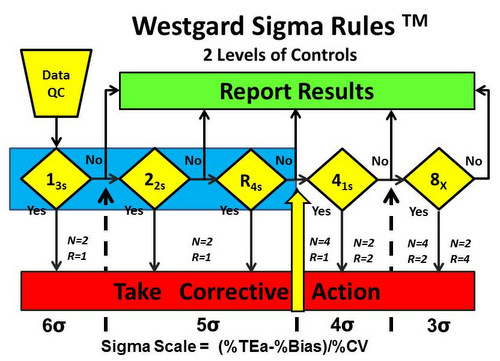
- 5-sigma quality requires 3 rules, 13s/22s/R4s, with 2 control measurements in each run (N=2, R=1). See Figure 3.
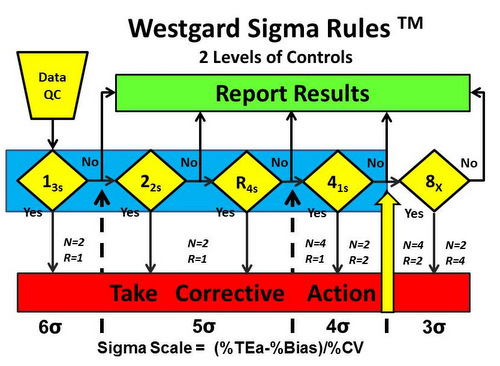
- 4-sigma quality requires addition of a 4th rule and implementation of a 13s/22s/R4s/41s multirole, preferably with 4 control measurements in each run (N=4, R=1), or alternatively, 2 control measurements in each of 2 runs (N=2, R=2), using the 41s rule to inspect the control rules across both runs. This 2nd option suggests dividing a day’s work into 2 runs and monitoring each with 2 controls. See Figure 4.
- <4-sigma quality requires a multirule procedure that includes the 8x rule, which can be implemented with 4 control measurements in each of 2 runs (N=4, R=2) or alternatively with 2 control measurements in each of 4 runs (N=2, N=4). The first option suggests dividing a days’ work into 2 runs with 4 control measurements per run, whereas the second option suggests dividing a day’s work into 4 runs and monitoring each with 2 controls. See Figure 5.
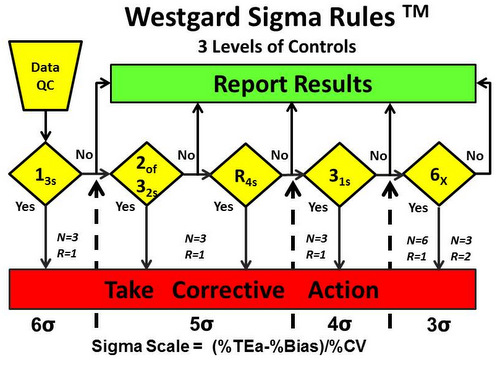
A similar diagram shown in Figure 6 describes Westgard Sigma Rules for 3 levels of controls.
- 6-sigma quality requires only a 13s rule and 1 measurement on each of 3 levels of controls. A
- 5-sigma quality requires adding the 2of32s and R4s rules for use with 1 measurement on each of 3 levels of controls.
- 4-sigma quality requires adding a 31s rule for use with 1 measurement on each of 3 controls.
- <4-sigma quality requires a multirule procedure that includes the 6x rule and a doubling of control measurements to a total of 6, which suggests that the 3 levels of controls be analyzed in duplicate in one run (N=6, R=1) or the day’s work divided into 2 runs with 3 control measurements per run (N=3, R=2). If a 9x rule were substituted for the 6x rule, then a day’s work could be divided into 3 runs with 3 controls per run (N=3,R=3).
What SQC is needed in the real world?
Based on data from which we have evaluated quality on the sigma scale, many highly automated systems provide a majority of tests that perform at 5 to 6 sigma quality. For those with 6-sigma quality, that means the use of a Levey-Jennings QC chart with control limits set as the mean ± 3SD and analysis of 2 controls per run should provide reliable detection of medically important errors. For those with 5 sigma quality, a simple multirule such as 13s/R4s/22s with 1 measurement on each of two levels of controls should be adequate. Such systems also typically include a few tests of lower quality which require more QC, with addition of the 41s and possibly the 8x rules and doubling the number of control measurements to provide a total N of 4.
In Point-of-Care applications, many test devices do not demonstrate high quality on the sigma scale, therefore the SQC required may be very demanding. As a specific example, the HbA1c devices that were evaluated by Lenters and Slingerland in the August 2014 issue of Clinical Chemistry [9] demonstrate sigma quality that ranges from approximately 0.0 to 4.5 (see discussion elsewhere on this website for the calculations). All 7 of these devices were NGSP certified as providing equivalent test results and furthermore classified by FDA as “waived” tests, meaning operators need no formal laboratory training, they only need to follow the manufacturer’s directions for QC, and the devices are not subject to ongoing assessment via proficiency testing. However, there is clearly a need for rigorous QC with use of many of these devices. Based on the sigma quality of these devices, the best case for SQC would be a multirule procedure 13s/22s/R4s/41s with 4 control measurements. The others require even more QC!
What’s the point?
Laboratories need to determine the sigma quality of their tests and methods if they are to manage the testing process properly. SQC is an essential tool for managing analytical quality, but the rules and number of control measurements should be optimized for quality and efficiency. It is very easy to figure out the right SQC by using Westgard Sigma Rules! The hard part is defining how good a test needs to be for its intended clinical use (e.g., the allowable Total Error, TEa), determining the precision (SD, CV) from a replication experiment or from routine SQC data, and determining accuracy (bias) from a comparison of methods experiment or from periodic proficiency testing results. After calculation of sigma quality, the Westgard Sigma Rules diagram makes it easy to select the right control rules and the right number of control measurements.
References
- Westgard JO, Groth T. Design and evaluation of statistical control procedures: Applications of a computer “Quality Control Simulator” program. Clin Chem 1981;27:1536-45.
- Westgard JO, Groth T. Power functions for statistical control rules. Clin Chem 1979;25:863-9.
- Koch DD, Oryall JJ, Quam EF, Feldbruegge, Dowd DE, Barry PL, Westgard JO. Selection of medically useful Quality Control procedures for individual tests done in a multitest analytical system. Clin Chem 1990;36:230-3.
- Westgard JO, Quam EF, Barry PL. Selection Grids for planning quality control procedures. Clin Lab Sci 1990;3:271-8.
- Westgard JO. Charts of Operational Process Specifications (OPSpecs Charts) for assessing the precision, accuracy, and quality control needed to satisfy proficiency testing criteria. Clin Chem 1992;38:1226-33.
- Westgard JO, Stein B. An automated process for selecting statistical QC procedures to assure clinical and analytical quality requirements. Clin Chem 1997;43:400-3.
- Westgard JO. Assuring the Right Quality Right. Chapter 11. How to use the EZRules 3 computer program. Madison WI:Westgard QC, Inc., 2007.
- Westgard JO, Westgard SA. Basic Quality Management Systems. Chapter 12. Designing SQC procedures. Madison WI:Westgard QC, Inc., 2014.
- Lenters-Westra E, Slingerland RJ. Three of 7 Hemoglobin A1c Point-of-Care instruments do not meet generally accepted analytical performance criteria. Clin Chem 2014;60:1062-1072.